当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Haber-independent, diversity-oriented synthesis of nitrogen compounds from biorenewable chitin
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0gc00208a Thuy Trang Pham 1, 2, 3, 4 , Xi Chen 5, 6, 7, 8 , Tilo Söhnel 1, 2, 3, 4 , Ning Yan 9, 10, 11 , Jonathan Sperry 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0gc00208a Thuy Trang Pham 1, 2, 3, 4 , Xi Chen 5, 6, 7, 8 , Tilo Söhnel 1, 2, 3, 4 , Ning Yan 9, 10, 11 , Jonathan Sperry 1, 2, 3, 4
Affiliation
|
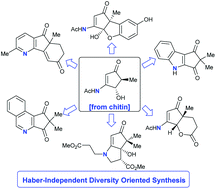
During drug discovery programmes, medicinal chemists rely on compounds derived from Haber ammonia as the source of this clinically important nitrogen heteroatom and as a result, the development of new pharmaceuticals is very energy intensive. Herein we report that biologically fixed nitrogen sourced from the biopolymer chitin can be readily incorporated into the diversity-oriented synthesis (DOS) of N-compounds relevant to drug discovery and development. The furfuryl alcohol derived from the chitin-sourced platform 3-acetamido-5-acetylfuran (3A5AF) undergoes a Piancatelli rearrangement to give a 3-aminocyclopentenone that is readily converted into a variety 4-aminocyclopentanones and 4-aminocyclopentene-1,3-diones. From two of these functionally rich bio-based platforms, structurally diverse, medicinally relevant N-scaffolds are attainable, including aminated Hajos–Parrish ketone (HPK) derivatives, aminated bicyclic ethers, bicyclic pyrrolidines, pyridines, isoquinolines and indoles. Thus, the DOS of compound libraries relevant to modern drug discovery programmes can be assembled independent of Haber ammonia.
中文翻译:

由生物可再生甲壳素独立于哈伯的,面向多样性的氮化合物的合成
在药物开发计划期间,药用化学家依靠源自哈伯氨的化合物作为这种临床上重要的氮杂原子的来源,因此,新药物的开发非常耗能。本文中,我们报道了源自生物聚合物几丁质的生物固定氮可以很容易地掺入与药物发现和开发相关的N化合物的面向多样性的合成(DOS)中。衍生自几丁质的平台3-乙酰氨基-5-乙酰基呋喃(3A5AF)的糠醇经过Piancatelli重排,生成3-氨基环戊烯酮,可轻松转化为各种4-氨基环戊酮和4-氨基环戊烯-1,3-二酮。从这两个功能丰富的生物基平台中,可以获得结构多样,具有医学相关性的N支架,包括胺化的Hajos-Parrish酮(HPK)衍生物,胺化的双环醚,双环吡咯烷,吡啶,异喹啉和吲哚。因此,与现代药物发现程序相关的化合物库的DOS可以独立于Haber氨水进行组装。
更新日期:2020-03-24
中文翻译:

由生物可再生甲壳素独立于哈伯的,面向多样性的氮化合物的合成
在药物开发计划期间,药用化学家依靠源自哈伯氨的化合物作为这种临床上重要的氮杂原子的来源,因此,新药物的开发非常耗能。本文中,我们报道了源自生物聚合物几丁质的生物固定氮可以很容易地掺入与药物发现和开发相关的N化合物的面向多样性的合成(DOS)中。衍生自几丁质的平台3-乙酰氨基-5-乙酰基呋喃(3A5AF)的糠醇经过Piancatelli重排,生成3-氨基环戊烯酮,可轻松转化为各种4-氨基环戊酮和4-氨基环戊烯-1,3-二酮。从这两个功能丰富的生物基平台中,可以获得结构多样,具有医学相关性的N支架,包括胺化的Hajos-Parrish酮(HPK)衍生物,胺化的双环醚,双环吡咯烷,吡啶,异喹啉和吲哚。因此,与现代药物发现程序相关的化合物库的DOS可以独立于Haber氨水进行组装。











































 京公网安备 11010802027423号
京公网安备 11010802027423号