当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peroxidation of 3,4-dihydro-1,4-benzoxazin-2-ones
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/26 , DOI: 10.1039/c9cc09778c Jie Wang 1, 2, 3, 4, 5 , Xiazhen Bao 1, 2, 3, 4, 5 , Jiayuan Wang 1, 2, 3, 4, 5 , Congde Huo 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/26 , DOI: 10.1039/c9cc09778c Jie Wang 1, 2, 3, 4, 5 , Xiazhen Bao 1, 2, 3, 4, 5 , Jiayuan Wang 1, 2, 3, 4, 5 , Congde Huo 1, 2, 3, 4, 5
Affiliation
|
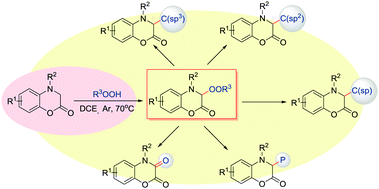
The sp3-C–H peroxidation of 3,4-dihydro-1,4-benzoxazin-2-ones was achieved under mild and simple catalyst-free reaction conditions. A range of biologically important alkylated benzoxazinone peroxides are synthesized in high yield with a good functional group tolerance. The C(sp3)-OO bond was constructed efficiently and could be further converted into C(sp3)–C(sp3), C(sp3)–C(sp2), C(sp3)–C(sp), C–P and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds by late-stage functional group transformations.
O bonds by late-stage functional group transformations.
中文翻译:

3,4-二氢-1,4-苯并恶嗪-2-酮的过氧化
3,4-二氢-1,4-苯并恶嗪-2-酮的sp 3 -C–H过氧化是在温和且简单的无催化剂条件下完成的。以高收率和良好的官能团耐受性合成了一系列生物学上重要的烷基化过氧化苯并恶嗪酮过氧化物。C(sp 3)-OO键结构有效,可以进一步转换为C(sp 3)–C(sp 3),C(sp 3)–C(sp 2),C(sp 3)–C( sp),C–P和C![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O键通过后期官能团转化而形成。
O键通过后期官能团转化而形成。
更新日期:2020-04-03
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds by late-stage functional group transformations.
O bonds by late-stage functional group transformations.
中文翻译:

3,4-二氢-1,4-苯并恶嗪-2-酮的过氧化
3,4-二氢-1,4-苯并恶嗪-2-酮的sp 3 -C–H过氧化是在温和且简单的无催化剂条件下完成的。以高收率和良好的官能团耐受性合成了一系列生物学上重要的烷基化过氧化苯并恶嗪酮过氧化物。C(sp 3)-OO键结构有效,可以进一步转换为C(sp 3)–C(sp 3),C(sp 3)–C(sp 2),C(sp 3)–C( sp),C–P和C
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) O键通过后期官能团转化而形成。
O键通过后期官能团转化而形成。







































 京公网安备 11010802027423号
京公网安备 11010802027423号