当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of Zaltoprofen in Five Binary Solvents at Various Temperatures: Data Determination and Thermodynamic Modeling
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.jced.9b01180 Renren Sun 1 , Yameng Wan 1 , Haixia He 1 , Jiao Sha 1 , Tao Li 1 , Baozeng Ren 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.jced.9b01180 Renren Sun 1 , Yameng Wan 1 , Haixia He 1 , Jiao Sha 1 , Tao Li 1 , Baozeng Ren 1
Affiliation
|
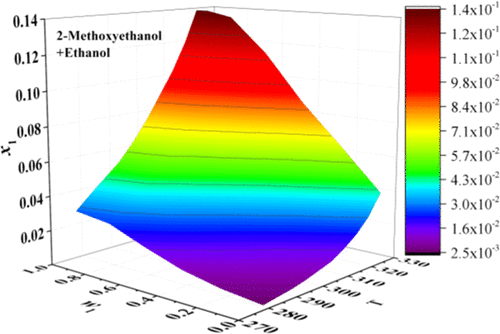
The solubility of zaltoprofen in binary {2-methoxyethanol (ME) + ethyl acetate (EAC)/ethanol (ET), 2-ethoxyethanol (EE) + ethyl acetate (EAC)} from 278.15 to 323.15 K and 1,4-dioxane (Diox) + ethyl acetate (EAC)/ethanol (ET) from 288.15 to 323.15 K was determined using the dynamic method under 0.1 MPa. The Hansen solubility parameter provided a reasonable explanation for the dissolution behavior of zaltoprofen. Three new crystal forms appeared during the dissolution process. Then, the Jouyban–Acree model, the Jouyban–Acree–van’t Hoff model, the Jouyban–Acree–Apelblat model, the Sun model, and the Ma model closely correlated with the measured solubility data. As a result, the Ma model and the Sun model provided better fitting. The basic data could provide support for industrial design and purification optimization process of zaltoprofen.
中文翻译:

扎托洛芬在五种二元溶剂中在不同温度下的溶解度:数据确定和热力学模型
扎托洛芬在二元{2-甲氧基乙醇(ME)+乙酸乙酯(EAC)/乙醇(ET),2-乙氧基乙醇(EE)+乙酸乙酯(EAC)}中的溶解度从278.15至323.15 K和1,4-二恶烷(使用动态方法在0.1 MPa下确定288.15至323.15 K的Diox)+乙酸乙酯(EAC)/乙醇(ET)。Hansen溶解度参数为扎洛特芬的溶解行为提供了合理的解释。在溶解过程中出现了三种新的晶体形式。然后,Jouyban-Acree模型,Jouyban-Acree-van't Hoff模型,Jouyban-Acree-Apelblat模型,Sun模型和Ma模型与测得的溶解度数据密切相关。结果,Ma模型和Sun模型提供了更好的拟合。该基础数据可为扎托洛芬的工业设计和纯化优化过程提供支持。
更新日期:2020-02-27
中文翻译:

扎托洛芬在五种二元溶剂中在不同温度下的溶解度:数据确定和热力学模型
扎托洛芬在二元{2-甲氧基乙醇(ME)+乙酸乙酯(EAC)/乙醇(ET),2-乙氧基乙醇(EE)+乙酸乙酯(EAC)}中的溶解度从278.15至323.15 K和1,4-二恶烷(使用动态方法在0.1 MPa下确定288.15至323.15 K的Diox)+乙酸乙酯(EAC)/乙醇(ET)。Hansen溶解度参数为扎洛特芬的溶解行为提供了合理的解释。在溶解过程中出现了三种新的晶体形式。然后,Jouyban-Acree模型,Jouyban-Acree-van't Hoff模型,Jouyban-Acree-Apelblat模型,Sun模型和Ma模型与测得的溶解度数据密切相关。结果,Ma模型和Sun模型提供了更好的拟合。该基础数据可为扎托洛芬的工业设计和纯化优化过程提供支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号