Oncogene ( IF 6.9 ) Pub Date : 2020-02-26 , DOI: 10.1038/s41388-020-1225-4 Appolinaire A Olou 1 , Ryan J King 1 , Fang Yu 2 , Pankaj K Singh 1, 2, 3, 4, 5
|
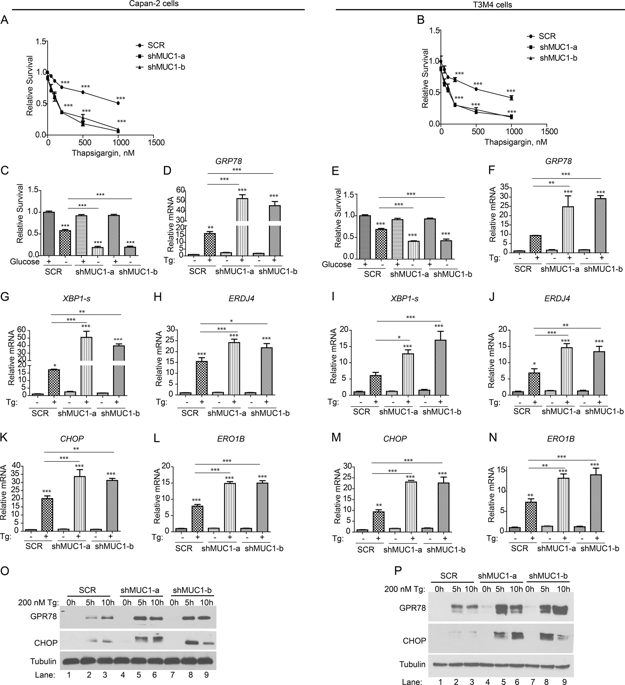
The Mucin 1 (MUC1) protein is overexpressed in various cancers and mediates chemotherapy resistance. However, the mechanism is not fully understood. Given that most chemotherapeutic drugs disrupt ER homeostasis as part of their toxicity, and MUC1 expression is regulated by proteins involved in ER homeostasis, we investigated the link between MUC1 and ER homeostasis. MUC1 knockdown in pancreatic cancer cells enhanced unfolded protein response (UPR) signaling and cell death upon ER stress induction. Transcriptomic analysis revealed alterations in the pyrimidine metabolic pathway and cytidine deaminase (CDA). ChIP and CDA activity assays showed that MUC1 occupied CDA gene promoter upon ER stress induction correlating with increased CDA expression and activity in MUC1-expressing cells as compared with MUC1 knockdown cells. Inhibition of either the CDA or pyrimidine metabolic pathway diminished survival in MUC1-expressing cancer cells upon ER stress induction. Metabolomic analysis demonstrated that MUC1-mediated CDA activity corresponded to deoxycytidine to deoxyuridine metabolic reprogramming upon ER stress induction. The resulting increase in deoxyuridine mitigated ER stress-induced cytotoxicity. In addition, given (1) the established roles of MUC1 in protecting cells against reactive oxygen species (ROS) insults, (2) ER stress-generated ROS further promote ER stress and (3) the emerging anti-oxidant property of deoxyuridine, we further investigated if MUC1 regulated ER stress by a deoxyuridine-mediated modulation of ROS levels. We observed that deoxyuridine could abrogate ROS-induced ER stress to promote cancer cell survival. Taken together, our findings demonstrate a novel MUC1-CDA axis of the adaptive UPR that provides survival advantage upon ER stress induction.
中文翻译:

MUC1癌蛋白通过CDA介导的嘧啶代谢重编程减轻ER应激
粘蛋白 1 (MUC1) 蛋白在各种癌症中过度表达并介导化疗耐药性。然而,该机制尚不完全清楚。鉴于大多数化疗药物会破坏 ER 稳态作为其毒性的一部分,并且 MUC1 的表达受参与 ER 稳态的蛋白质的调节,我们研究了 MUC1 和 ER 稳态之间的联系。胰腺癌细胞中的 MUC1 敲低增强了 ER 应激诱导后的未折叠蛋白反应 (UPR) 信号传导和细胞死亡。转录组学分析揭示了嘧啶代谢途径和胞苷脱氨酶 (CDA) 的改变。ChIP 和 CDA 活性测定表明,与 MUC1 敲低细胞相比,MUC1 在 ER 应激诱导后占据 CDA 基因启动子,这与 MUC1 表达细胞中 CDA 表达和活性增加相关。抑制 CDA 或嘧啶代谢途径会降低表达 MUC1 的癌细胞在 ER 应激诱导后的存活率。代谢组学分析表明,MUC1 介导的 CDA 活性对应于 ER 应激诱导后的脱氧胞苷到脱氧尿苷代谢重编程。由此产生的脱氧尿苷增加减轻了 ER 应激诱导的细胞毒性。此外,鉴于(1)MUC1 在保护细胞免受活性氧(ROS)损伤方面的既定作用,(2)ER 应激产生的 ROS 进一步促进 ER 应激和(3)脱氧尿苷新兴的抗氧化特性,我们进一步研究了 MUC1 是否通过脱氧尿苷介导的 ROS 水平调节来调节 ER 应激。我们观察到脱氧尿苷可以消除 ROS 诱导的 ER 应激以促进癌细胞存活。综合起来,











































 京公网安备 11010802027423号
京公网安备 11010802027423号