Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.elecom.2020.106690 Zhaoping Shi , Xin Li , Tongfei Li , Yifan Chen , Yawen Tang
|
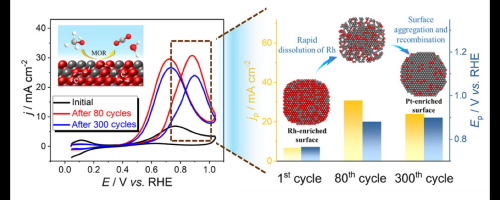
PtRh alloys are highly active in the anodic methanol oxidation reaction (MOR) but few studies have looked at their evolution in the composition and structure during the catalytic process. Herein, a uniformly dispersed carbon-supported PtRh (PtRh/C) catalyst with Rh-enriched surface was synthesized via a facial hydrothermal method. By recording the performance of different cyclic voltammetry (CV) cycles during electrocatalytic MOR process in acid, the evolution of PtRh/C in composition and structure was elucidated. During the first 80 CV cycles, the rapid dissolution of the surface Rh atoms and the exposure of active Pt sites lead to the dramatic enhancement of the current density. When further continue the CV cycling, Pt atoms on the surface have aggregated and recombined to form a tightly aligned Pt-enriched surface. But Rh tends to be stable and catalytic performance reaches a relatively stable status with slightly decreased activity. Compared with the performance of PtRu/C catalyst, it can be concluded that PtRh/C has better stability and higher activity based on the less soluble property of Rh than Ru.
中文翻译:

酸性甲醇电氧化过程中PtRh / C的组成和结构的演变
PtRh合金在阳极甲醇氧化反应(MOR)中具有很高的活性,但是很少有研究关注其在催化过程中的组成和结构演变。在此,通过面部水热法合成了表面富含Rh的均匀分散的碳担载的PtRh(PtRh / C)催化剂。通过记录在酸中电催化MOR过程中不同循环伏安(CV)循环的性能,阐明了PtRh / C在组成和结构上的演变。在最初的80个CV循环中,表面Rh原子的快速溶解和活性Pt部位的暴露导致电流密度的显着提高。当进一步继续CV循环时,表面上的Pt原子聚集并重新结合,形成紧密排列的富含Pt的表面。但是Rh趋于稳定,催化性能达到相对稳定的状态,活性略有下降。与PtRu / C催化剂的性能相比,可以得出结论,由于Rh的溶解性低于Ru,因此PtRh / C具有更好的稳定性和更高的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号