当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1-(2-bromo-1-arylethyl)-1H-benzotriazoles via NBS promoted addition of 1H-benzotriazole to alkene: Relevance in benzotriazole ring cleavage
Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.tet.2020.131078 Mala Singh , Priyanka Bose , Anoop S. Singh , Vinod K. Tiwari
中文翻译:

通过NBS合成1-(2-溴-1-芳基乙基)-1 H-苯并三唑促进了1 H-苯并三唑向烯烃的加成:苯并三唑环裂解的相关性
更新日期:2020-02-27
Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.tet.2020.131078 Mala Singh , Priyanka Bose , Anoop S. Singh , Vinod K. Tiwari
|
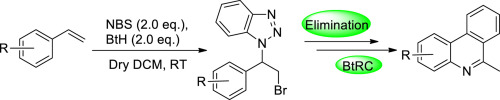
A cost-effective and expeditious one-pot synthesis of 1-(2-bromo-1-arylethyl)-1H-benzotriazoles has been devised by reacting styrene and their derivatives with 1H-benzotriazole in presence of N-bromosuccinimide in anhydrous CH2Cl2 at room temperature. Further, the resulted compounds undergo E2-ellimination to afford the respective 1-(1-aryl-vinyl)-1H-benzotriazoles. At the end, 1-(1-phenylvinyl)-1H-benzotriazoles underwent benzotriazole ring cleavage (BtRC) under free radical condition to produce phenanthridine as the final product.
中文翻译:

通过NBS合成1-(2-溴-1-芳基乙基)-1 H-苯并三唑促进了1 H-苯并三唑向烯烃的加成:苯并三唑环裂解的相关性
通过使苯乙烯及其衍生物与1 H-苯并三唑在N-溴代琥珀酰亚胺存在下于无水CH中反应,已设计出一种经济高效的一锅法合成1-(2-溴-1-芳基乙基)-1 H-苯并三唑2 Cl 2在室温下。此外,将所得的化合物进行E 2-消除,以提供相应的1-(1-芳基-乙烯基)-1 H-苯并三唑。最后,在自由基条件下对1-(1-苯基乙烯基)-1 H-苯并三唑进行苯并三唑环裂解(BtRC),以产生菲啶作为最终产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号