当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Creation of glycoprotein imprinted self-assembled monolayers with dynamic boronate recognition sites and imprinted cavities for selective glycoprotein recognition.
Soft Matter ( IF 2.9 ) Pub Date : 2020-03-04 , DOI: 10.1039/c9sm02313e Xianfeng Zhang 1 , Xuezhong Du
Soft Matter ( IF 2.9 ) Pub Date : 2020-03-04 , DOI: 10.1039/c9sm02313e Xianfeng Zhang 1 , Xuezhong Du
Affiliation
|
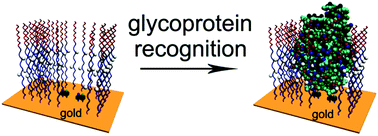
Glycoproteins are involved in the pathogenesis and development of many diseases and are used as biomarkers for disease diagnosis. It is highly desirable to develop highly sensitive and selective methods for the detection of glycoproteins without the use of antibodies. Imprinting of proteins represents one of the most challenging tasks. Glycoprotein imprinted self-assembled monolayers (SAMs) were created, for the first time, from an oligo(ethylene glycol) (OEG) terminated 1,2-dithiolane derivative linked through an alkyl chain incorporated with two amide groups (DHAP) and combined functional thiols of p-mercaptophenylboronic acid (PMBA) and p-aminothiophenol (PATP) in aqueous media, without the use of polymerization initiators. Combined action of PMBA and PATP was essential for the development of boronate recognition sites for glycoproteins at the physiological pH, attributed to the water molecule-mediated Lewis acid-base interactions between the electron-deficient PMBA and the electron-rich PATP. DHAP played key roles not only in cementation of imprinted cavities by means of double hydrogen bond networks through the amide groups but also in resistance to nonspecific protein binding by terminal OEG moieties, as well as hydrogen bond binding sites from the amide groups exposed to imprinted cavities. The created glycoprotein imprinted SAMs showed excellent recognition selectivity of target glycoproteins. The strategy for tailor-made glycoprotein imprinted SAMs explores a new avenue to the creation of intelligent biomaterials and fabrication of chemosensors.
中文翻译:

糖蛋白印迹自组装单层的创建,具有动态的硼酸盐识别位点和用于选择性糖蛋白识别的印迹腔。
糖蛋白参与许多疾病的发病和发展,并被用作疾病诊断的生物标志物。非常需要开发不使用抗体来检测糖蛋白的高度灵敏和选择性的方法。蛋白质的印迹是最具挑战性的任务之一。糖蛋白印迹自组装单分子层(SAMs)首次由寡聚乙二醇(OEG)封端的1,2-二硫杂环戊烷衍生物形成,该衍生物通过与两个酰胺基(DHAP)结合的烷基链连接并结合在一起对-巯基苯基硼酸(PMBA)和对-氨基苯硫酚(PATP)的硫醇,无需使用聚合引发剂。PMBA和PATP的联合作用对于在生理pH值下糖蛋白的硼酸酯识别位点的开发至关重要,这归因于缺电子的PMBA与富电子的PATP之间的水分子介导的Lewis酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。归因于缺电子的PMBA与富电子的PATP之间的水分子介导的路易斯酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。归因于缺电子的PMBA与富电子的PATP之间的水分子介导的路易斯酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中发挥了关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥了关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。所创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。
更新日期:2020-03-26
中文翻译:

糖蛋白印迹自组装单层的创建,具有动态的硼酸盐识别位点和用于选择性糖蛋白识别的印迹腔。
糖蛋白参与许多疾病的发病和发展,并被用作疾病诊断的生物标志物。非常需要开发不使用抗体来检测糖蛋白的高度灵敏和选择性的方法。蛋白质的印迹是最具挑战性的任务之一。糖蛋白印迹自组装单分子层(SAMs)首次由寡聚乙二醇(OEG)封端的1,2-二硫杂环戊烷衍生物形成,该衍生物通过与两个酰胺基(DHAP)结合的烷基链连接并结合在一起对-巯基苯基硼酸(PMBA)和对-氨基苯硫酚(PATP)的硫醇,无需使用聚合引发剂。PMBA和PATP的联合作用对于在生理pH值下糖蛋白的硼酸酯识别位点的开发至关重要,这归因于缺电子的PMBA与富电子的PATP之间的水分子介导的Lewis酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。归因于缺电子的PMBA与富电子的PATP之间的水分子介导的路易斯酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。归因于缺电子的PMBA与富电子的PATP之间的水分子介导的路易斯酸碱相互作用。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中发挥了关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥了关键作用。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。DHAP不仅在通过酰胺基团通过双氢键网络胶结印迹腔的过程中起着关键作用,而且在抵抗末端OEG部分的非特异性蛋白质结合以及暴露于印迹腔的酰胺基团的氢键结合位点方面发挥着关键作用。所创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。创建的糖蛋白印迹SAM显示出对目标糖蛋白的出色识别选择性。量身定制的糖蛋白印迹SAM的策略探索了创建智能生物材料和化学传感器制造的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号