当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed carbonylative transformation of aryl iodides and sulfonyl chlorides: convenient access to thioesters
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0qo00158a Xinxin Qi 1, 2, 3, 4 , Zhi-Peng Bao 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/26 , DOI: 10.1039/d0qo00158a Xinxin Qi 1, 2, 3, 4 , Zhi-Peng Bao 1, 2, 3, 4 , Xiao-Feng Wu 1, 2, 3, 4, 5
Affiliation
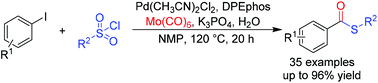
|
A palladium-catalyzed carbonylative transformation of aryl iodides and sulfonyl chlorides has been studied. Both aryl and alkyl sulfonyl chlorides were all successfully transformed into the desired thioesters with sulfonyl chlorides as the sulfur source. Mo(CO)6 played a dual role as both CO surrogate and reductant in this reaction.
中文翻译:

钯催化的芳基碘化物和磺酰氯的羰基化转化:方便地获得硫酯
已经研究了钯催化的芳基碘化物和磺酰氯的羰基化转化。以磺酰氯为硫源,芳基和烷基磺酰氯均成功地转化为所需的硫酯。Mo(CO)6在该反应中同时扮演着CO替代物和还原剂的双重角色。
更新日期:2020-03-19
中文翻译:

钯催化的芳基碘化物和磺酰氯的羰基化转化:方便地获得硫酯
已经研究了钯催化的芳基碘化物和磺酰氯的羰基化转化。以磺酰氯为硫源,芳基和烷基磺酰氯均成功地转化为所需的硫酯。Mo(CO)6在该反应中同时扮演着CO替代物和还原剂的双重角色。









































 京公网安备 11010802027423号
京公网安备 11010802027423号