当前位置:
X-MOL 学术
›
Environ. Sci.: Processes Impacts
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoisomer specific reaction of hexabromocyclododecane with Fe(ii) associated with iron oxides.
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-04-29 , DOI: 10.1039/c9em00587k Xianmiao Zhang 1 , Kristian K Roopnarine , Shirley Dong , Urs Jans
Environmental Science: Processes & Impacts ( IF 4.3 ) Pub Date : 2020-04-29 , DOI: 10.1039/c9em00587k Xianmiao Zhang 1 , Kristian K Roopnarine , Shirley Dong , Urs Jans
Affiliation
|
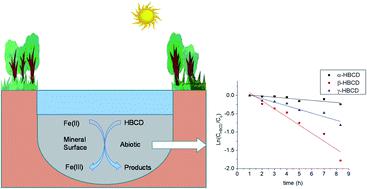
The reactions of hexabromocyclododecane (HBCD) isomers with Fe(ii) associated with iron oxides were performed in a pH range from 6.15 to 7.50 at room temperature. It was observed that Fe(ii) associated with iron oxides (i.e., goethite, magnetite, hematite) is a better reductant than just an aqueous solution of Fe(ii) to potentially reduce HBCD in subsurface environments. The reaction of HBCD with Fe(ii) associated with iron oxides is also stereoisomer specific with α-HBCD reacting much slower than β-HBCD and γ-HBCD. The reaction is pH dependent and it is faster with increased pH. The initial concentration of Fe(ii) and HBCD can also affect the reaction rate. The reaction is negligible when all the Fe(ii) is sorbed to magnetite and no Fe(ii) remains dissolved. It was also observed that the reaction of 100 nM HBCD is slower than the reaction of 1.0 μM HBCD with Fe(ii) associated with magnetite. In addition, natural organic matter (NOM) was found to inhibit the degradation of HBCD by Fe(ii) associated with iron oxides.
中文翻译:

六溴环十二烷与与氧化铁缔合的Fe(ii)的立体异构体特异性反应。
六溴环十二烷(HBCD)异构体与Fe(ii)与氧化铁缔合的反应在室温下于6.15至7.50的pH范围内进行。据观察,与铁氧化物(即针铁矿,磁铁矿,赤铁矿)缔合的Fe(ii)是一种更好的还原剂,其效果不仅限于Fe(ii)的水溶液,还可能在地下环境中还原六溴环十二烷。六溴环十二烷与与氧化铁缔合的Fe(ii)的反应也是立体异构体特有的,α-六溴环十二烷的反应比β-六溴环十二烷和γ-六溴环十二烷的反应要慢得多。该反应是pH依赖性的,并且随着pH的升高反应更快。Fe(ii)和HBCD的初始浓度也会影响反应速率。当所有的Fe(ii)都吸附到磁铁矿上并且没有Fe(ii)保持溶解时,该反应可以忽略不计。还观察到100 nM HBCD的反应比1。0μM六溴环十二烷,其中Fe(ii)与磁铁矿结合。此外,发现天然有机物(NOM)可以抑制与氧化铁相关的Fe(ii)对六溴环十二烷的降解。
更新日期:2020-02-25
中文翻译:

六溴环十二烷与与氧化铁缔合的Fe(ii)的立体异构体特异性反应。
六溴环十二烷(HBCD)异构体与Fe(ii)与氧化铁缔合的反应在室温下于6.15至7.50的pH范围内进行。据观察,与铁氧化物(即针铁矿,磁铁矿,赤铁矿)缔合的Fe(ii)是一种更好的还原剂,其效果不仅限于Fe(ii)的水溶液,还可能在地下环境中还原六溴环十二烷。六溴环十二烷与与氧化铁缔合的Fe(ii)的反应也是立体异构体特有的,α-六溴环十二烷的反应比β-六溴环十二烷和γ-六溴环十二烷的反应要慢得多。该反应是pH依赖性的,并且随着pH的升高反应更快。Fe(ii)和HBCD的初始浓度也会影响反应速率。当所有的Fe(ii)都吸附到磁铁矿上并且没有Fe(ii)保持溶解时,该反应可以忽略不计。还观察到100 nM HBCD的反应比1。0μM六溴环十二烷,其中Fe(ii)与磁铁矿结合。此外,发现天然有机物(NOM)可以抑制与氧化铁相关的Fe(ii)对六溴环十二烷的降解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号