当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel class of benzimidazole-thiazole hybrids: The privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.bmc.2020.115403 Mohammed T-E Maghraby 1 , Ola M F Abou-Ghadir 1 , Samia G Abdel-Moty 1 , Asmaa Y Ali 2 , Ola I A Salem 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.bmc.2020.115403 Mohammed T-E Maghraby 1 , Ola M F Abou-Ghadir 1 , Samia G Abdel-Moty 1 , Asmaa Y Ali 2 , Ola I A Salem 1
Affiliation
|
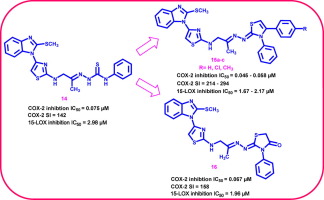
The present study includes design and synthesis of new molecular hybrids of 2-methylthiobenzimidazole linked to various anti-inflammatory pharmacophores through 2-aminothiazole linker, to investigate the effect of such molecular variation on cyclooxygenase (COX) and 15-lipoxygenase (15-LOX) enzymes inhibition as well as in vivo anti-inflammatory activity. The chemical structures of new hybrids were confirmed using different spectroscopic tools and elemental analyses. Benzimidazole-thiazole hybrids linked to acetyl moiety 13, phenyl thiosemicarbazone 14, 1,3-thiazolines 15a-c and 4-thiazolidinone 16 exhibited significant COX-2 inhibition (IC50 = 0.045-0.075 µM) with significant COX-2 selectivity indices (SI = 142-294). All hybrids revealed potent 15-LOX inhibitory activity (IC50 = 1.67-6.56 µM). Benzimidazole-thiazole hybrid 15b was the most potent dual COX-2 (IC50 = 0.045 µM, SI = 294) inhibitor approximate to celecoxib (COX-2; IC50 = 0.045 µM, SI = 327), with double inhibitory activity versus 15-LOX enzyme (IC50 = 1.67 µM) relative to quercetin (IC50 = 3.34 µM). Three hybrids (14, 15b &16) were selected for in vivo screening using carrageenan-induced paw edema method. Benzimidazole-thiazole hybrid linked to 4-thiazolidinone 16 showed the maximum edema inhibition at both 3 h and 4 h intervals as well (~119% and 102% relative to indomethacin, respectively). The gastric ulcerogenic effect of benzimidazole-thiazole hybrid 16 was estimated compared with indomethacin showing superior gastrointestinal safety profile. In bases of molecular modeling; all new active hybrids were subjected to docking simulation into active sites of COX-2 and 15-LOX enzymes to study the binding mode of these novel potent dual COX-2/15-LOX inhibitors.
中文翻译:

新型苯并咪唑-噻唑杂化物:具有强大的抗炎活性,同时具有双重抑制环氧合酶和15-脂氧合酶的特权支架。
本研究包括设计和合成2-甲基硫代苯并咪唑通过2-氨基噻唑接头与各种消炎药效团连接的新型分子杂合体,以研究这种分子变异对环氧合酶(COX)和15-脂氧合酶(15-LOX)的影响。酶抑制以及体内抗炎活性。使用不同的光谱工具和元素分析确认了新杂种的化学结构。与乙酰基部分13,苯基硫代半碳酰胺14、1,3-噻唑啉15a-c和4-噻唑烷酮16连接的苯并咪唑-噻唑杂化物表现出显着的COX-2抑制作用(IC50 = 0.045-0.075 µM),并具有明显的COX-2选择性指数(SI) = 142-294)。所有杂种均显示出有效的15-LOX抑制活性(IC50 = 1.67-6.56 µM)。苯并咪唑-噻唑杂化物15b是最有效的双重COX-2抑制剂(IC50 = 0.045 µM,SI = 294),近似于塞来昔布(COX-2; IC50 = 0.045 µM,SI = 327),与15-LOX相比具有双重抑制活性相对于槲皮素(IC50 = 3.34 µM)的酶(IC50 = 1.67 µM)。使用角叉菜胶诱导的爪水肿方法,选择了三个杂种(14、15b和16)进行体内筛选。与4-噻唑烷酮16连接的苯并咪唑-噻唑杂化物在3 h和4 h间隔也显示出最大的水肿抑制作用(相对于消炎痛分别为119%和102%)。与吲哚美辛相比,苯并咪唑-噻唑杂化物16的胃溃疡作用得到了评估,显示出优越的胃肠道安全性。以分子建模为基础;
更新日期:2020-02-26
中文翻译:

新型苯并咪唑-噻唑杂化物:具有强大的抗炎活性,同时具有双重抑制环氧合酶和15-脂氧合酶的特权支架。
本研究包括设计和合成2-甲基硫代苯并咪唑通过2-氨基噻唑接头与各种消炎药效团连接的新型分子杂合体,以研究这种分子变异对环氧合酶(COX)和15-脂氧合酶(15-LOX)的影响。酶抑制以及体内抗炎活性。使用不同的光谱工具和元素分析确认了新杂种的化学结构。与乙酰基部分13,苯基硫代半碳酰胺14、1,3-噻唑啉15a-c和4-噻唑烷酮16连接的苯并咪唑-噻唑杂化物表现出显着的COX-2抑制作用(IC50 = 0.045-0.075 µM),并具有明显的COX-2选择性指数(SI) = 142-294)。所有杂种均显示出有效的15-LOX抑制活性(IC50 = 1.67-6.56 µM)。苯并咪唑-噻唑杂化物15b是最有效的双重COX-2抑制剂(IC50 = 0.045 µM,SI = 294),近似于塞来昔布(COX-2; IC50 = 0.045 µM,SI = 327),与15-LOX相比具有双重抑制活性相对于槲皮素(IC50 = 3.34 µM)的酶(IC50 = 1.67 µM)。使用角叉菜胶诱导的爪水肿方法,选择了三个杂种(14、15b和16)进行体内筛选。与4-噻唑烷酮16连接的苯并咪唑-噻唑杂化物在3 h和4 h间隔也显示出最大的水肿抑制作用(相对于消炎痛分别为119%和102%)。与吲哚美辛相比,苯并咪唑-噻唑杂化物16的胃溃疡作用得到了评估,显示出优越的胃肠道安全性。以分子建模为基础;










































 京公网安备 11010802027423号
京公网安备 11010802027423号