Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.tet.2020.131079 Máté Gergely , Attila Bényei , László Kollár
|
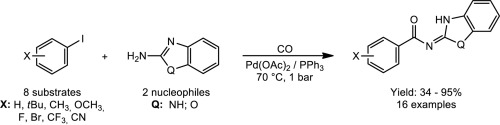
Palladium-catalysed aminocarbonylation of aryl iodides in the presence of 2-aminobenzimidazole and 2-aminobenzoxazole as N-nucleophile was carried out. Single CO insertion took place, however, instead of the expected carboxamides (C(O)NH) the corresponding N-acyl-imine (C(O)NC) derivatives were obtained. The structure of the latter compounds can be explained by tautomerization involving the heterocyclic ring. The above structures without amide-NH moieties were proved by methylation at the NH groups of the heterocycle. The resulted mono- and dimethylated benzimidazole derivatives, as well as monomethylated benzoxazole derivatives, like the parent N-acylated compounds, were fully characterised including single crystal X-ray crystallography.
中文翻译:

2-氨基苯并咪唑和-苯并恶唑作为N-亲核试剂在钯催化的氨基羰基化反应中
在2-氨基苯并咪唑和2-氨基苯并恶唑作为N-亲核试剂的存在下,钯催化芳基碘的氨基羰基化反应。仅发生一次CO插入,但是获得了相应的N-酰基亚胺(C(O)N C)衍生物,而不是预期的羧酰胺(C(O)NH)。后一种化合物的结构可以通过涉及杂环的互变异构来解释。通过在杂环的NH基上甲基化证明了以上没有酰胺-NH部分的结构。所得的单和二甲基化的苯并咪唑衍生物,以及单甲基化的苯并恶唑衍生物,如母体N-酰化的化合物,被充分表征,包括单晶X射线晶体学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号