Organic Electronics ( IF 2.7 ) Pub Date : 2020-02-26 , DOI: 10.1016/j.orgel.2020.105688 Gyounglyul Jo , Seung Hyun Cho , Hyungwoo Kim , Hyeonseok Yoon , Sangil Han , Mincheol Chang
|
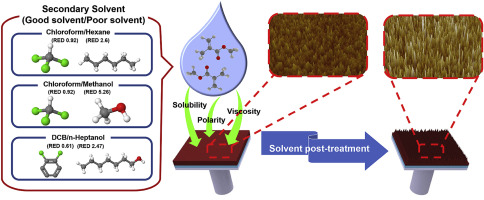
Secondary solvents are utilized in post-processing procedures, such as the solvent post-treatment and post-deposition of polymer protecting and dielectric layers, and their effects on the morphology, molecular ordering, and charge-transport characteristics of poly (3-hexylthiophene) (P3HT) thin films have been investigated by systematically varying the polarity, viscosity, and solubility of co-solvent systems (i.e., chloroform/hexane, chloroform/methanol, and DCB/n-heptanol). During the solvent post-treatment, the P3HT films are preferentially dissolved by a co-solvent in the following order: DCB/n-heptanol < chloroform/methanol < chloroform/hexane co-solvent system. The chloroform/hexane blend with a lower polarity preferably dissolved the P3HT films compared to the chloroform/methanol blend with a higher polarity owing to a better compatibility with non-polar hexyl side chains of P3HT. The chloroform/methanol blend, which has a relatively low viscosity, was found to be more effective for the dissolution of the P3HT films than the DCB/n-heptanol blend, which has a high viscosity, owing to its faster diffusion and better wettability. Interestingly, it was revealed that co-solvent systems increasingly facilitate the rearrangement of P3HT chains with a decrease in the RED values (i.e., increase in solubility), resulting in enhanced intra- and intermolecular interactions of P3HT films. Meanwhile, the crystal grain size of the P3HT films did not noticeably vary. Consequently, as the relative energy difference (RED) values of secondary co-solvents decreased, the average mobility of the P3HT films increased in the following order: DCB/n-heptanol < chloroform/methanol < chloroform/hexane. Further, we apply secondary solvents to the post-deposition of polymer protecting layers and dielectric layers, and study their effects on the conjugated polymer films. In contrast with the case involving solvent post-treatment, the secondary co-solvents used in the case of the post-deposition of either a polymer protecting or dielectric layer (i.e., poly (methyl methacrylate) (PMMA)) very slightly affected the molecular ordering, morphology, and charge-transport properties of the P3HT films for solvent RED values ranging from 1.2 to 1.8; this is because of the attenuated interactions between the solvent and P3HT molecules by the PMMA molecules, which indicates that the spectrum of secondary solvent systems and polymers that are applicable to the deposition of protecting polymer and dielectric layers can be expanded.
中文翻译:

辅助溶剂对共轭聚合物薄膜的形态和电荷传输的影响
辅助溶剂用于后处理程序中,例如聚合物保护和介电层的溶剂后处理和后沉积,以及它们对聚(3-己基噻吩)的形态,分子有序性和电荷传输特性的影响(P3HT)薄膜已通过系统地改变助溶剂体系(即氯仿/己烷,氯仿/甲醇和DCB /正庚醇)的极性,粘度和溶解度进行了研究。在溶剂后处理过程中,P3HT膜优先按以下顺序被共溶剂溶解:DCB /正庚醇<氯仿/甲醇<氯仿/己烷共溶剂体系。与具有较高极性的氯仿/甲醇共混物相比,具有较低极性的氯仿/己烷共混物优选溶解P3HT膜,这是由于与P3HT的非极性己基侧链更好的相容性。已发现,具有较低粘度的氯仿/甲醇混合物比具有较高粘度的DCB /正庚醇混合物对P3HT膜的溶解更有效,这归因于其更快的扩散和更好的润湿性。有趣的是,发现助溶剂系统越来越促进P3HT链的重排,而RED值降低(即,溶解度增加),导致P3HT膜的分子内和分子间相互作用增强。同时,P3HT膜的晶粒尺寸没有明显变化。所以,随着辅助助溶剂相对能量差(RED)值的降低,P3HT膜的平均迁移率按以下顺序增加:DCB /正庚醇<氯仿/甲醇<氯仿/己烷。此外,我们将二次溶剂应用于聚合物保护层和介电层的后沉积,并研究它们对共轭聚合物薄膜的影响。与涉及溶剂后处理的情况相比,在聚合物保护层或介电层(即,聚(甲基丙烯酸甲酯)(PMMA))的后沉积情况下使用的辅助助溶剂对分子的影响很小。对于溶剂RED值在1.2到1.8之间的P3HT薄膜,其有序,形态和电荷传输特性











































 京公网安备 11010802027423号
京公网安备 11010802027423号