当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of ex vivo catabolites of peptides with doping potential in equine plasma by HILIC-HRMS.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-03-24 , DOI: 10.1002/dta.2781 Fuyu Guan 1, 2 , Savannah Fay 1, 2 , Xiaoqing Li 1, 2 , Youwen You 1, 2 , Mary A Robinson 1, 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-03-24 , DOI: 10.1002/dta.2781 Fuyu Guan 1, 2 , Savannah Fay 1, 2 , Xiaoqing Li 1, 2 , Youwen You 1, 2 , Mary A Robinson 1, 2
Affiliation
|
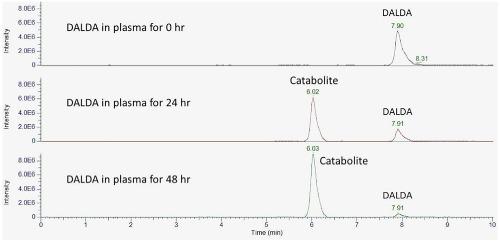
Bioactive peptides pose a great threat to sports integrity. The detection of these peptides is essential for enforcing their prohibition in sports. Identifying the catabolites of these peptides that are formed ex vivo in plasma may improve their detection. In the present study, the stability of 27 bioactive peptides with protection at both termini in equine plasma was examined under different incubation conditions, using HILIC coupled to HRMS. Of the 27 peptides, 13 were stable after incubation at 37°C for 72 hr, but the remaining 14 were less stable. Ex vivo catabolites of these 14 peptides were detected using their theoretical masses generated in silico, their appearance was monitored over the time course of incubation, and their identity was verified by their product ion spectra. Catabolites identified for chemotactic peptide, DALDA, dmtDALDA, deltorphins I and II, Hyp6‐dermorphin, Lys7‐dermorphin, and dermorphin analog are novel. A d‐amino acid residue at position 2 or 1 of a peptide or next to its C‐terminus protected the relevant terminal from degradation by exopeptidases, but such a residue at position 3 did not. A pGlu residue or N‐methylation at the N‐terminus of a peptide did not protect its N‐terminal. Ethylamide at the C‐terminus of a peptide provided the C‐terminal protection from attacks by carboxypeptidases. The C‐terminal Lys amide in DALDA, dmtDALDA, and Lys7‐dermorphin was susceptible to cleavage by plasma enzymes, which is the first report, to the authors’ knowledge. The results from the present study provide insights into the stability of peptides in plasma.
中文翻译:

通过HILIC-HRMS鉴定马血浆中具有掺杂潜力的肽离体分解代谢产物。
生物活性肽对运动的完整性构成巨大威胁。这些肽的检测对于在运动中强制禁止它们是必不可少的。鉴定在血浆中离 体形成的这些肽的分解代谢物可以改善其检测。在本研究中,使用HILIC偶联HRMS,在不同的孵育条件下检查了马血浆中两个末端具有保护作用的27种生物活性肽的稳定性。在27种肽中,有13种在37°C温育72小时后稳定,但其余14种不稳定。È X 体内使用他们的理论质量产生检测这14种肽的分解代谢产物,在硅片,在孵育的整个过程中对它们的外观进行监测,并通过其产物离子光谱验证其身份。为趋化肽,DALDA,dmtDALDA,deltorphins I和II,Hyp 6- dermorphin,Lys 7- dermorphin和dermorphin类似物鉴定的分解代谢物是新颖的。肽第2或1位或其C端附近的d-氨基酸残基保护相关末端不被外肽酶降解,但第3位的此类残基却没有。肽N末端的pGlu残基或N甲基化不能保护其N末端。肽C末端的乙酰胺可保护C末端免受羧肽酶攻击。DALDA,dmtDALDA和Lys 7中的C末端Lys酰胺据作者所知,这是第一份报告,血浆吗啡易被血浆酶裂解。本研究的结果为深入了解血浆中肽的稳定性提供了见识。
更新日期:2020-03-24
中文翻译:

通过HILIC-HRMS鉴定马血浆中具有掺杂潜力的肽离体分解代谢产物。
生物活性肽对运动的完整性构成巨大威胁。这些肽的检测对于在运动中强制禁止它们是必不可少的。鉴定在血浆中离 体形成的这些肽的分解代谢物可以改善其检测。在本研究中,使用HILIC偶联HRMS,在不同的孵育条件下检查了马血浆中两个末端具有保护作用的27种生物活性肽的稳定性。在27种肽中,有13种在37°C温育72小时后稳定,但其余14种不稳定。È X 体内使用他们的理论质量产生检测这14种肽的分解代谢产物,在硅片,在孵育的整个过程中对它们的外观进行监测,并通过其产物离子光谱验证其身份。为趋化肽,DALDA,dmtDALDA,deltorphins I和II,Hyp 6- dermorphin,Lys 7- dermorphin和dermorphin类似物鉴定的分解代谢物是新颖的。肽第2或1位或其C端附近的d-氨基酸残基保护相关末端不被外肽酶降解,但第3位的此类残基却没有。肽N末端的pGlu残基或N甲基化不能保护其N末端。肽C末端的乙酰胺可保护C末端免受羧肽酶攻击。DALDA,dmtDALDA和Lys 7中的C末端Lys酰胺据作者所知,这是第一份报告,血浆吗啡易被血浆酶裂解。本研究的结果为深入了解血浆中肽的稳定性提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号