当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Epigallocatechin-3-gallate Inhibits Cu(II)-Induced β-2-Microglobulin Amyloid Formation by Binding to the Edge of Its β-Sheets.
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.biochem.0c00043 Tyler M Marcinko 1 , Thomas Drews 1 , Tianying Liu 1 , Richard W Vachet 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.biochem.0c00043 Tyler M Marcinko 1 , Thomas Drews 1 , Tianying Liu 1 , Richard W Vachet 1
Affiliation
|
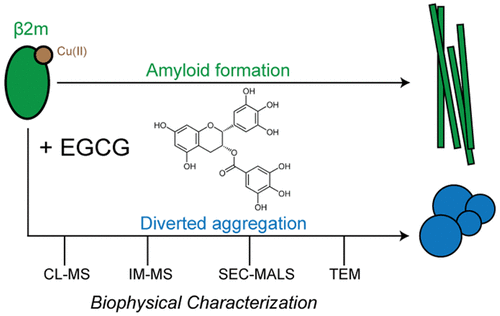
Epigallocatechin-3-gallate (EGCG) is a catechin found in green tea that can inhibit the amyloid formation of a wide variety of proteins. EGCG's ability to prevent or redirect the amyloid formation of so many proteins may reflect a common mechanism of action, and thus, greater molecular-level insight into how it exerts its effect could have broad implications. Here, we investigate the molecular details of EGCG's inhibition of the protein β-2-microglobulin (β2m), which forms amyloids in patients undergoing long-term dialysis treatment. Using size-exclusion chromatography and a collection of mass spectrometry-based techniques, we find that EGCG prevents Cu(II)-induced β2m amyloid formation by diverting the normal progression of preamyloid oligomers toward the formation of spherical, redissolvable aggregates. EGCG exerts its effect by binding with a micromolar affinity (Kd ≈ 5 μM) to the β2m monomer on the edge of two β-sheets near the N-terminus. This interaction destabilizes the preamyloid dimer and prevents the formation of a tetramer species previously shown to be essential for Cu(II)-induced β2m amyloid formation. EGCG's binding at the edge of the β-sheets in β2m is consistent with a previous hypothesis that EGCG generally prevents amyloid formation by binding cross-β-sheet aggregation intermediates.
中文翻译:

Epigallocatechin-3-gallate通过与β-Sheets边缘结合来抑制Cu(II)诱导的β-2-微球蛋白淀粉样蛋白形成。
Epigallocatechin-3-gallate(EGCG)是在绿茶中发现的一种儿茶素,可以抑制多种蛋白质的淀粉样蛋白形成。EGCG阻止或重定向许多蛋白质的淀粉样蛋白形成的能力可能反映了一种共同的作用机制,因此,对其作用如何发挥更大的分子水平的了解可能具有广泛的意义。在这里,我们研究了EGCG抑制蛋白β-2-微球蛋白(β2m)的分子细节,该蛋白在接受长期透析治疗的患者中会形成淀粉样蛋白。使用尺寸排阻色谱法和基于质谱的技术集合,我们发现EGCG通过将淀粉样前低聚物的正常进展转向球形,可再溶解的聚集体来阻止Cu(II)诱导的β2m淀粉样蛋白形成。EGCG通过与微摩尔亲和力(Kd≈5μM)与靠近N末端的两个β-折叠边缘上的β2m单体结合而发挥作用。这种相互作用破坏了淀粉样前体的二聚体的稳定性,并阻止了四聚体物种的形成,该四聚体物种先前对于Cu(II)诱导的β2m淀粉样蛋白的形成至关重要。EGCG在β2m中的β-折叠边缘处的结合与先前的假设一致,即EGCG通常通过结合交叉的β-折叠聚集中间体来防止淀粉样蛋白形成。
更新日期:2020-03-04
中文翻译:

Epigallocatechin-3-gallate通过与β-Sheets边缘结合来抑制Cu(II)诱导的β-2-微球蛋白淀粉样蛋白形成。
Epigallocatechin-3-gallate(EGCG)是在绿茶中发现的一种儿茶素,可以抑制多种蛋白质的淀粉样蛋白形成。EGCG阻止或重定向许多蛋白质的淀粉样蛋白形成的能力可能反映了一种共同的作用机制,因此,对其作用如何发挥更大的分子水平的了解可能具有广泛的意义。在这里,我们研究了EGCG抑制蛋白β-2-微球蛋白(β2m)的分子细节,该蛋白在接受长期透析治疗的患者中会形成淀粉样蛋白。使用尺寸排阻色谱法和基于质谱的技术集合,我们发现EGCG通过将淀粉样前低聚物的正常进展转向球形,可再溶解的聚集体来阻止Cu(II)诱导的β2m淀粉样蛋白形成。EGCG通过与微摩尔亲和力(Kd≈5μM)与靠近N末端的两个β-折叠边缘上的β2m单体结合而发挥作用。这种相互作用破坏了淀粉样前体的二聚体的稳定性,并阻止了四聚体物种的形成,该四聚体物种先前对于Cu(II)诱导的β2m淀粉样蛋白的形成至关重要。EGCG在β2m中的β-折叠边缘处的结合与先前的假设一致,即EGCG通常通过结合交叉的β-折叠聚集中间体来防止淀粉样蛋白形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号