当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differential effects of heat-inactivated, secretome-deficient MSC and metabolically active MSC in sepsis and allogenic heart transplantation
STEM CELLS ( IF 4.0 ) Pub Date : 2020-03-12 , DOI: 10.1002/stem.3165 Andreas R R Weiss 1, 2 , Olivia Lee 3, 4 , Elke Eggenhofer 1 , Elisabeth Geissler 1 , Sander S Korevaar 3 , Yorick Soeder 1, 5 , Hans J Schlitt 1 , Edward K Geissler 1 , Martin J Hoogduijn 3 , Marc H Dahlke 1, 5
STEM CELLS ( IF 4.0 ) Pub Date : 2020-03-12 , DOI: 10.1002/stem.3165 Andreas R R Weiss 1, 2 , Olivia Lee 3, 4 , Elke Eggenhofer 1 , Elisabeth Geissler 1 , Sander S Korevaar 3 , Yorick Soeder 1, 5 , Hans J Schlitt 1 , Edward K Geissler 1 , Martin J Hoogduijn 3 , Marc H Dahlke 1, 5
Affiliation
|
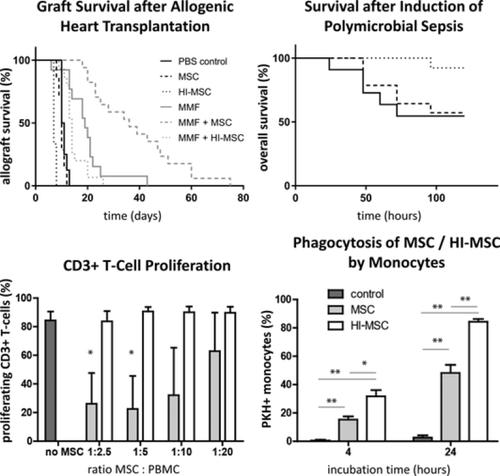
Mesenchymal stem cells (MSCs) are used in various clinical and preclinical models for immunomodulation. However, it remains unclear how the immunomodulatory effect of MSC is communicated. MSC‐induced immunomodulation is known to be mediated through both MSC‐secreted cytokines and direct cell‐cell interactions. Recently, it has been demonstrated that metabolically inactive, heat‐inactivated MSCs (HI‐MSCs) have similar anti‐inflammatory capacities in LPS‐induced sepsis compared with viable MSC. To further investigate the immunomodulatory effects of MSC, we introduced MSC and HI‐MSC in two animal models with different immunological causes. In the first model, allogeneic hearts were transplanted from C57BL/6 mice to BALB/c recipients. MSC in combination with mycophenolate mofetil (MMF) significantly improved graft survival compared with MMF alone, whereas the application of HI‐MSC had no effect on graft survival. We revealed that control MSC dose‐dependently inhibited CD3+ and CD8+ T‐cell proliferation in vitro, whereas HI‐MSC had no effect. In the second model, sepsis was induced in mice via cecal ligation and puncture. HI‐MSC treatment significantly improved the overall survival, whereas control MSCs had no effect. in vitro studies demonstrated that HI‐MSCs are more effectively phagocytosed by monocytes than control MSCs and induced cell death in particular of activated CD16+ monocytes, which may explain the immune protective effect of HI‐MSC in the sepsis model. The results of our study demonstrate that MSC‐mediated immunomodulation in sepsis is dependent on a passive recognition of MSC by monocytes, whereas fully functional MSCs are required for inhibition of T‐cell‐mediated allograft rejection.
中文翻译:

热灭活、分泌组缺陷的 MSC 和代谢活跃的 MSC 在败血症和同种异体心脏移植中的不同作用
间充质干细胞 (MSCs) 用于免疫调节的各种临床和临床前模型。然而,目前尚不清楚 MSC 的免疫调节作用是如何传递的。已知 MSC 诱导的免疫调节是通过 MSC 分泌的细胞因子和直接的细胞-细胞相互作用介导的。最近,已经证明代谢失活、热灭活的 MSCs (HI-MSCs) 在 LPS 诱导的败血症中具有与存活的 MSCs 相似的抗炎能力。为了进一步研究 MSC 的免疫调节作用,我们在具有不同免疫学原因的两种动物模型中引入了 MSC 和 HI-MSC。在第一个模型中,同种异体心脏从 C57BL/6 小鼠移植到 BALB/c 受体。与单独使用 MMF 相比,MSC 与霉酚酸酯 (MMF) 联合使用可显着提高移植物存活率,而HI-MSC的应用对移植物存活没有影响。我们发现对照 MSC 在体外剂量依赖性地抑制 CD3+ 和 CD8+ T 细胞增殖,而 HI-MSC 没有效果。在第二个模型中,通过盲肠结扎和穿刺在小鼠中诱导败血症。HI-MSC 治疗显着提高了总体存活率,而对照 MSC 没有效果。体外研究表明,HI-MSCs 比对照 MSCs 更有效地被单核细胞吞噬并诱导细胞死亡,尤其是活化的 CD16+单核细胞,这可能解释了 HI-MSC 在败血症模型中的免疫保护作用。我们的研究结果表明,脓毒症中 MSC 介导的免疫调节依赖于单核细胞对 MSC 的被动识别,
更新日期:2020-03-12
中文翻译:

热灭活、分泌组缺陷的 MSC 和代谢活跃的 MSC 在败血症和同种异体心脏移植中的不同作用
间充质干细胞 (MSCs) 用于免疫调节的各种临床和临床前模型。然而,目前尚不清楚 MSC 的免疫调节作用是如何传递的。已知 MSC 诱导的免疫调节是通过 MSC 分泌的细胞因子和直接的细胞-细胞相互作用介导的。最近,已经证明代谢失活、热灭活的 MSCs (HI-MSCs) 在 LPS 诱导的败血症中具有与存活的 MSCs 相似的抗炎能力。为了进一步研究 MSC 的免疫调节作用,我们在具有不同免疫学原因的两种动物模型中引入了 MSC 和 HI-MSC。在第一个模型中,同种异体心脏从 C57BL/6 小鼠移植到 BALB/c 受体。与单独使用 MMF 相比,MSC 与霉酚酸酯 (MMF) 联合使用可显着提高移植物存活率,而HI-MSC的应用对移植物存活没有影响。我们发现对照 MSC 在体外剂量依赖性地抑制 CD3+ 和 CD8+ T 细胞增殖,而 HI-MSC 没有效果。在第二个模型中,通过盲肠结扎和穿刺在小鼠中诱导败血症。HI-MSC 治疗显着提高了总体存活率,而对照 MSC 没有效果。体外研究表明,HI-MSCs 比对照 MSCs 更有效地被单核细胞吞噬并诱导细胞死亡,尤其是活化的 CD16+单核细胞,这可能解释了 HI-MSC 在败血症模型中的免疫保护作用。我们的研究结果表明,脓毒症中 MSC 介导的免疫调节依赖于单核细胞对 MSC 的被动识别,











































 京公网安备 11010802027423号
京公网安备 11010802027423号