Biosensors and Bioelectronics ( IF 10.7 ) Pub Date : 2020-02-25 , DOI: 10.1016/j.bios.2020.112112 Ieva Plikusiene , Zigmas Balevicius , Almira Ramanaviciene , Julian Talbot , Gitana Mickiene , Saulius Balevicius , Arunas Stirke , Alla Tereshchenko , Linas Tamosaitis , Gintautas Zvirblis , Arunas Ramanavicius
|
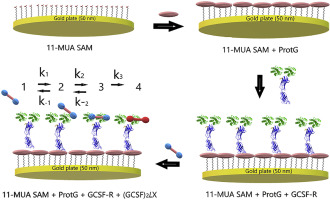
The modelling of protein-protein binding kinetics is important for the development of affinity-sensors and the prediction of signaling protein based drug efficiency. Therefore, in this research we have evaluated the binding kinetics of several genetically designed protein models: (i) three different ligands based on granulocyte colony-stimulating factor GCSF homo-dimeric derivatives linked by differed by linkers of different length and flexibility; (ii) an antibody-like receptor (GCSF-R) based on two GCSF-receptor sites immobilized to Fc domains, which are common parts of protein structures forming antibodies. Genetically engineered GCSF-R is similar to an antibody because it, like the antibody, has two binding sites, which both selectively bind with GCSF ligands. To design the affinity sensor model studied here, GCSF-R was immobilized on a thin gold layer via self-assembled monolayer conjugated with Protein-G. Binding kinetics between immobilized GCSF-R and all three different recombinant GCSF-based homo-dimeric derivatives were evaluated by total internal reflection ellipsometry. Association constants were determined by fitting mathematical models to the experimental data. It was clearly observed that both (i) affinity and (ii) binding kinetics depend on the length and flexibility of the linker that connects both domains of a GCSF-based ligand. The fastest association between immobilized GCSF-R and GCSF-based ligands was observed for ligands whose GCSF domains were interconnected by the longest and the most flexible linker. Here we present ellipsometry-based measurements and models of the interaction kinetics that advance the understanding of bidentate-receptor-based immunosensor action and enables us to predict the optimal linker structure for the design of GCSF-based medications.
中文翻译:

评估亲和力传感器对二聚体配体的反应动力学与不同刚性的间隔基连接:固定的重组粒细胞集落刺激因子合成受体与基因工程二聚体分析物衍生物的结合
蛋白质-蛋白质结合动力学的建模对于开发亲和传感器和预测基于信号的蛋白质的药物效率非常重要。因此,在这项研究中,我们评估了几种遗传设计的蛋白质模型的结合动力学:(i)基于粒细胞集落刺激因子GCSF同型二聚体衍生物的三种不同配体,其通过不同长度和柔性的连接子而不同;(ii)基于固定在Fc域上的两个GCSF受体位点的抗体样受体(GCSF-R),这是形成抗体的蛋白质结构的常见部分。基因改造的GCSF-R与抗体相似,因为它与抗体一样,具有两个结合位点,两个结合位点均选择性地与GCSF配体结合。要设计此处研究的亲和力传感器模型,通过自组装的单层蛋白G。通过全内反射椭圆光度法评估固定的GCSF-R与所有三种不同的基于重组GCSF的同型二聚体衍生物之间的结合动力学。通过将数学模型拟合到实验数据来确定缔合常数。清楚地观察到,(i)亲和力和(ii)结合动力学都取决于连接基于GCSF的配体的两个结构域的接头的长度和柔性。对于其GCSF结构域通过最长和最灵活的连接子相互连接的配体,观察到固定的GCSF-R和基于GCSF的配体之间最快的关联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号