Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SUMO system - a key regulator in sarcomere organization.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-02-25 , DOI: 10.1111/febs.15263 Arnab Nayak 1 , Mamta Amrute-Nayak 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-02-25 , DOI: 10.1111/febs.15263 Arnab Nayak 1 , Mamta Amrute-Nayak 1
Affiliation
|
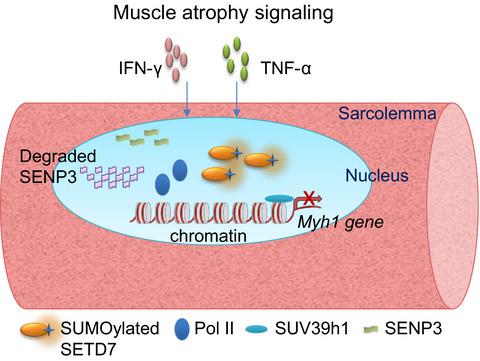
Skeletal muscles constitute roughly 40% of human body mass. Muscles are specialized tissues that generate force to drive movements through ATP‐driven cyclic interactions between the protein filaments, namely actin and myosin filaments. The filaments are organized in an intricate structure called the ‘sarcomere’, which is a fundamental contractile unit of striated skeletal and cardiac muscle, hosting a fine assembly of macromolecular protein complexes. The micrometer‐sized sarcomere units are arranged in a reiterated array within myofibrils of muscle cells. The precise spatial organization of sarcomere is tightly controlled by several molecular mechanisms, indispensable for its force‐generating function. Disorganized sarcomeres, either due to erroneous molecular signaling or due to mutations in the sarcomeric proteins, lead to human diseases such as cardiomyopathies and muscle atrophic conditions prevalent in cachexia. Protein post‐translational modifications (PTMs) of the sarcomeric proteins serve a critical role in sarcomere formation (sarcomerogenesis), as well as in the steady‐state maintenance of sarcomeres. PTMs such as phosphorylation, acetylation, ubiquitination, and SUMOylation provide cells with a swift and reversible means to adapt to an altered molecular and therefore cellular environment. Over the past years, SUMOylation has emerged as a crucial modification with implications for different aspects of cell function, including organizing higher‐order protein assemblies. In this review, we highlight the fundamentals of the small ubiquitin‐like modifiers (SUMO) pathway and its link specifically to the mechanisms of sarcomere assembly. Furthermore, we discuss recent studies connecting the SUMO pathway‐modulated protein homeostasis with sarcomere organization and muscle‐related pathologies.
中文翻译:

SUMO系统-肌节组织中的关键调节器。
骨骼肌约占人体质量的40%。肌肉是专门的组织,通过蛋白质细丝(即肌动蛋白和肌球蛋白细丝)之间由ATP驱动的循环相互作用产生力来驱动运动。细丝以称为“肌节”的复杂结构组织,这是横纹骨骼肌和心肌的基本收缩单位,具有大分子蛋白质复合物的精细组装。微米级的肌小节单位在肌肉细胞的肌原纤维中以重复的阵列排列。肌节的精确空间组织受到多种分子机制的严格控制,这是其产生力的功能所不可缺少的。杂乱的肉瘤,可能是由于错误的分子信号传导或由于肌节蛋白的突变,导致人类疾病,例如恶病质中普遍存在的心肌病和肌肉萎缩症。肌节蛋白的蛋白质翻译后修饰(PTM)在肌节的形成(肉瘤发生)以及肉瘤的稳态维持中起着关键作用。PTM(例如磷酸化,乙酰化,泛素化和SUMO酰化)为细胞提供了迅速而可逆的方式,以适应分子和细胞环境的变化。在过去的几年中,SUMOylation已作为一种至关重要的修饰形式出现,对细胞功能的各个方面都具有影响,包括组织更高级别的蛋白质装配。在这篇综述中,我们着重介绍了小泛素样修饰剂(SUMO)途径的基本原理,以及它与肌节组装机制的特殊联系。此外,
更新日期:2020-02-25
中文翻译:

SUMO系统-肌节组织中的关键调节器。
骨骼肌约占人体质量的40%。肌肉是专门的组织,通过蛋白质细丝(即肌动蛋白和肌球蛋白细丝)之间由ATP驱动的循环相互作用产生力来驱动运动。细丝以称为“肌节”的复杂结构组织,这是横纹骨骼肌和心肌的基本收缩单位,具有大分子蛋白质复合物的精细组装。微米级的肌小节单位在肌肉细胞的肌原纤维中以重复的阵列排列。肌节的精确空间组织受到多种分子机制的严格控制,这是其产生力的功能所不可缺少的。杂乱的肉瘤,可能是由于错误的分子信号传导或由于肌节蛋白的突变,导致人类疾病,例如恶病质中普遍存在的心肌病和肌肉萎缩症。肌节蛋白的蛋白质翻译后修饰(PTM)在肌节的形成(肉瘤发生)以及肉瘤的稳态维持中起着关键作用。PTM(例如磷酸化,乙酰化,泛素化和SUMO酰化)为细胞提供了迅速而可逆的方式,以适应分子和细胞环境的变化。在过去的几年中,SUMOylation已作为一种至关重要的修饰形式出现,对细胞功能的各个方面都具有影响,包括组织更高级别的蛋白质装配。在这篇综述中,我们着重介绍了小泛素样修饰剂(SUMO)途径的基本原理,以及它与肌节组装机制的特殊联系。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号