当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Spectroscopic Studies on the Redox Cycle of NH3−SCR over Cu−CHA Zeolites
ChemCatChem ( IF 3.8 ) Pub Date : 2020-02-25 , DOI: 10.1002/cctc.202000024 Chong Liu 1 , Hiroe Kubota 1 , Takehiro Amada 1 , Kenichi Kon 1 , Takashi Toyao 1, 2 , Zen Maeno 1 , Kakuya Ueda 3 , Junya Ohyama 2, 4 , Atsushi Satsuma 2, 3 , Takuya Tanigawa 5 , Nao Tsunoji 5 , Tsuneji Sano 5 , Ken‐ichi Shimizu 1, 2
ChemCatChem ( IF 3.8 ) Pub Date : 2020-02-25 , DOI: 10.1002/cctc.202000024 Chong Liu 1 , Hiroe Kubota 1 , Takehiro Amada 1 , Kenichi Kon 1 , Takashi Toyao 1, 2 , Zen Maeno 1 , Kakuya Ueda 3 , Junya Ohyama 2, 4 , Atsushi Satsuma 2, 3 , Takuya Tanigawa 5 , Nao Tsunoji 5 , Tsuneji Sano 5 , Ken‐ichi Shimizu 1, 2
Affiliation
|
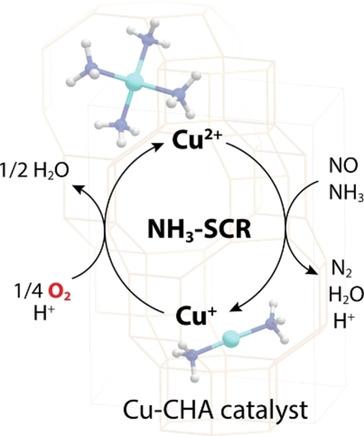
The selective catalytic reduction of NO with ammonia (NH3−SCR) catalyzed by Cu−CHA zeolites is thoroughly investigated using in situ spectroscopic experiments combined with on‐line mass spectroscopy (MS) under steady‐state NH3−SCR conditions and transient conditions for Cu(II)/Cu(I) redox cycles. Quantitative analysis of the in situ XANES spectra of Cu−CHA under steady‐state conditions of NH3−SCR show that NH3‐coordinated Cu(II) species is the dominant Cu species at low temperatures (100–150 °C). At higher temperatures, Cu(II) species and [Cu(NH3)2]+ complex coexist, possibly because the rate of the Cu(II)→Cu(I) reduction step is comparable to that of the Cu(I)→Cu(II) oxidation step. In situ XANES, IR/MS, and UV‐vis/MS experiments on the reduction half cycle demonstrate that the reduction of Cu(II) species occurs via the reaction of NH3‐liganded Cu(II) with NO to yield N2 and H2O. For the oxidation half cycle, in situ XANES experiments of Cu(I) oxidation in 10 % O2 at 200 °C indicate that an increased density in CHA zeolite exhibits a higher oxidation rate. In situ UV‐vis experiments of Cu(I) reoxidation using different mixtures of oxidant feed gas demonstrate the key role of O2 in the oxidation cycle. It is suggested that the reoxidation of Cu(I) to Cu(II) species occurs with only O2 as the oxidant, and a high Cu density in CHA zeolite promotes SCR activity by enhancing the oxidative activation of Cu(I) to Cu(II) during the catalytic cycle.
中文翻译:

Cu-CHA沸石上NH3-SCR氧化还原循环的原位光谱研究
在稳态NH 3 -SCR条件和瞬态条件下,结合原位光谱实验和在线质谱(MS),对Cu-CHA沸石催化的氨选择性催化还原NO(NH 3 -SCR)进行了研究。用于Cu(II)/ Cu(I)氧化还原循环。在稳态NH 3 -SCR条件下,Cu-CHA的原位XANES光谱定量分析显示,在低温(100-150°C)下,NH 3配位的Cu(II)物种是主要的Cu物种。在较高温度下,Cu(II)和[Cu(NH 3)2 ] +络合物并存,可能是因为Cu(II)→Cu(I)还原步骤的速率与Cu(I)→Cu(II)氧化步骤的速率相当。还原半衰期的原位XANES,IR / MS和UV-vis / MS实验表明,Cu(II)物种的还原是通过NH 3配位的Cu(II)与NO反应生成N 2和ħ 2 O.用于氧化半周期中,在原位在10%氧气的Cu的XANES实验(I)氧化2在200℃下表明,CHA沸石的增加的密度表现出更高的氧化速率。使用氧化剂进料气的不同混合物对Cu(I)进行再氧化的原位UV-vis实验证明了O 2的关键作用在氧化循环中。建议仅以O 2为氧化剂发生Cu(I)到Cu(II)物种的再氧化,CHA沸石中的高Cu密度通过增强Cu(I)到Cu(I)的氧化活化而促进SCR活性。 II)在催化循环中。
更新日期:2020-02-25
中文翻译:

Cu-CHA沸石上NH3-SCR氧化还原循环的原位光谱研究
在稳态NH 3 -SCR条件和瞬态条件下,结合原位光谱实验和在线质谱(MS),对Cu-CHA沸石催化的氨选择性催化还原NO(NH 3 -SCR)进行了研究。用于Cu(II)/ Cu(I)氧化还原循环。在稳态NH 3 -SCR条件下,Cu-CHA的原位XANES光谱定量分析显示,在低温(100-150°C)下,NH 3配位的Cu(II)物种是主要的Cu物种。在较高温度下,Cu(II)和[Cu(NH 3)2 ] +络合物并存,可能是因为Cu(II)→Cu(I)还原步骤的速率与Cu(I)→Cu(II)氧化步骤的速率相当。还原半衰期的原位XANES,IR / MS和UV-vis / MS实验表明,Cu(II)物种的还原是通过NH 3配位的Cu(II)与NO反应生成N 2和ħ 2 O.用于氧化半周期中,在原位在10%氧气的Cu的XANES实验(I)氧化2在200℃下表明,CHA沸石的增加的密度表现出更高的氧化速率。使用氧化剂进料气的不同混合物对Cu(I)进行再氧化的原位UV-vis实验证明了O 2的关键作用在氧化循环中。建议仅以O 2为氧化剂发生Cu(I)到Cu(II)物种的再氧化,CHA沸石中的高Cu密度通过增强Cu(I)到Cu(I)的氧化活化而促进SCR活性。 II)在催化循环中。









































 京公网安备 11010802027423号
京公网安备 11010802027423号