当前位置:
X-MOL 学术
›
J. Chromatogr. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immobilized metal affinity chromatography matrix modified by poly (ethylene glycol) methyl ether for purification of angiotensin I-converting enzyme inhibitory peptide from casein hydrolysate.
Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-02-25 , DOI: 10.1016/j.jchromb.2020.122042 Pengru Liu 1 , Xiongdiao Lan 2 , Muhammad Yaseen 3 , Kungang Chai 4 , Liqin Zhou 4 , Jianhua Sun 4 , Ping Lan 2 , Zhangfa Tong 4 , Dankui Liao 4 , Lixia Sun 4
Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-02-25 , DOI: 10.1016/j.jchromb.2020.122042 Pengru Liu 1 , Xiongdiao Lan 2 , Muhammad Yaseen 3 , Kungang Chai 4 , Liqin Zhou 4 , Jianhua Sun 4 , Ping Lan 2 , Zhangfa Tong 4 , Dankui Liao 4 , Lixia Sun 4
Affiliation
|
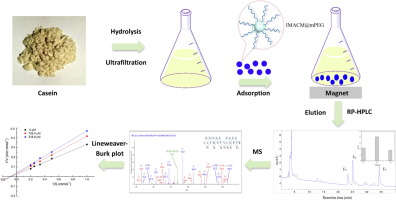
Purification of small bioactive peptides from complex biological samples is a difficult task due to the interference of concentrated large biomolecules. In this study, a magnetic immobilized metal affinity chromatography matrix modified by poly (ethylene glycol) methyl ether (IMACM@mPEG) was prepared and applied for the rapid purification of angiotensin I-converting enzyme (ACE) inhibitory peptides from casein hydrolysate. The proposed IMACM@mPEG considerably reduced the non-specific adsorption of large proteins and exhibited improved purification efficiency towards ACE inhibitory peptides. A novel peptide with moderate ACE inhibitory activity (IC50 value of 274 ± 5 μM) was identified as LLYQEPVLGPVR. Lineweaver-Burk plot confirmed the non-competitive inhibition pattern of LLYQEPVLGPVR. The purified peptide was digested after simulated gastrointestinal digestion and produced shorter peptides which contributed to enhanced ACE inhibitory activity. These results indicated that the IMACM@mPEG is an effective method for the prepurification of ACE inhibitory peptide and the purified peptide LLYQEPVLGPVR may have potential as nutraceutical ingredient in functional foods for hypertension treatments.
中文翻译:

固定化的金属亲和层析基质,经聚(乙二醇)甲醚改性,可从酪蛋白水解物中纯化血管紧张素I转化酶抑制肽。
由于浓缩的大生物分子的干扰,从复杂的生物样品中纯化小生物活性肽是一项艰巨的任务。在这项研究中,制备了一种由聚(乙二醇)甲醚(IMACM @ mPEG)修饰的磁性固定金属亲和色谱基质,并将其用于从酪蛋白水解产物中快速纯化血管紧张素I转化酶(ACE)抑制肽。提出的IMACM @ mPEG大大减少了大蛋白的非特异性吸附,并表现出对ACE抑制肽的更高的纯化效率。具有中等ACE抑制活性(IC50值为274±5μM)的新型肽被鉴定为LLYQEPVLGPVR。Lineweaver-Burk图证实了LLYQEPVLGPVR的非竞争抑制模式。模拟胃肠道消化后,将纯化的肽消化,并产生较短的肽,这有助于增强ACE抑制活性。这些结果表明,IMACM @ mPEG是一种预纯化ACE抑制肽的有效方法,而纯化的肽LLYQEPVLGPVR可能作为高血压治疗功能性食品中的营养成分。
更新日期:2020-02-25
中文翻译:

固定化的金属亲和层析基质,经聚(乙二醇)甲醚改性,可从酪蛋白水解物中纯化血管紧张素I转化酶抑制肽。
由于浓缩的大生物分子的干扰,从复杂的生物样品中纯化小生物活性肽是一项艰巨的任务。在这项研究中,制备了一种由聚(乙二醇)甲醚(IMACM @ mPEG)修饰的磁性固定金属亲和色谱基质,并将其用于从酪蛋白水解产物中快速纯化血管紧张素I转化酶(ACE)抑制肽。提出的IMACM @ mPEG大大减少了大蛋白的非特异性吸附,并表现出对ACE抑制肽的更高的纯化效率。具有中等ACE抑制活性(IC50值为274±5μM)的新型肽被鉴定为LLYQEPVLGPVR。Lineweaver-Burk图证实了LLYQEPVLGPVR的非竞争抑制模式。模拟胃肠道消化后,将纯化的肽消化,并产生较短的肽,这有助于增强ACE抑制活性。这些结果表明,IMACM @ mPEG是一种预纯化ACE抑制肽的有效方法,而纯化的肽LLYQEPVLGPVR可能作为高血压治疗功能性食品中的营养成分。









































 京公网安备 11010802027423号
京公网安备 11010802027423号