当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drug Permeation against Efflux by Two Transporters.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-02-25 , DOI: 10.1021/acsinfecdis.9b00510 Paramita Saha 1 , Samapan Sikdar 1 , Ganesh Krishnamoorthy 1 , Helen I Zgurskaya 1 , Valentin V Rybenkov 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-02-25 , DOI: 10.1021/acsinfecdis.9b00510 Paramita Saha 1 , Samapan Sikdar 1 , Ganesh Krishnamoorthy 1 , Helen I Zgurskaya 1 , Valentin V Rybenkov 1
Affiliation
|
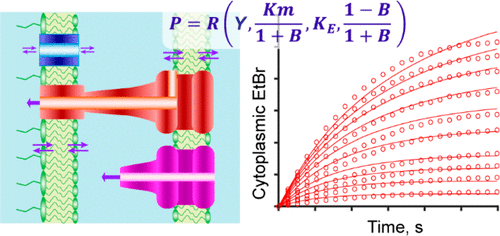
The development of new antibiotics against Gram-negative bacteria is hampered by the powerful protective properties of their cell envelope. This envelope consists of two membranes augmented by efflux transporters, which act in synergy to restrict cellular access to a broad range of chemical compounds. Recently, a kinetic model of this system has been constructed. The model revealed a complex, nonlinear behavior of the system, complete with a bifurcation, and matched very well to experimental uptake data. Here, we expand the model to include multiple transporters and apply it to an experimental analysis of antibiotic accumulation in wild-type and efflux-deficient Escherichia coli. We show that transporters acting across the inner and outer membranes have synergistic effects with each other. In contrast, transporters acting across the same membrane are additive as a rule but can be synergistic under special circumstances owing to a bifurcation controlled by the barrier constant. With respect to ethidium bromide, the inner membrane transporter MdfA was synergistic to the TolC-dependent efflux across the outer membrane. The agreement between the model and drug accumulation was very good across a range of tested drug concentrations and strains. However, antibiotic susceptibilities related only qualitatively to the accumulation of the drugs or predictions of the model and could be fit to the model only if additional assumptions were made about the physiological consequences of prolonged cell exposure to the drugs. Thus, the constructed model correctly predicts transmembrane permeation of various compounds and potentially their intracellular activity.
中文翻译:

两种转运蛋白对外排的药物渗透。
抗革兰氏阴性细菌的新抗生素的开发受到其细胞膜强大的保护特性的阻碍。该包膜由两个由外排转运蛋白增强的膜组成,外排转运蛋白协同作用以限制细胞接触多种化学物质。最近,已经建立了该系统的动力学模型。该模型揭示了系统的复杂,非线性行为,具有分叉,并且与实验吸收数据非常吻合。在这里,我们将模型扩展为包括多个转运蛋白,并将其应用于野生型和外排缺陷型大肠杆菌中抗生素积累的实验分析。我们表明,跨内膜和外膜起作用的转运蛋白彼此具有协同作用。相反,跨同一膜起作用的转运蛋白通常是加性的,但由于势垒常数控制的分叉,在特殊情况下可能具有协同作用。关于溴化乙锭,内膜转运蛋白MdfA与穿过外膜的TolC依赖性外排协同作用。在一系列测试药物浓度和菌株中,模型与药物积累之间的一致性非常好。但是,抗生素敏感性仅在质量上与药物的积累或模型的预测有关,并且只有在对延长的细胞长期暴露于药物的生理后果做出其他假设的情况下,抗生素敏感性才可能与模型相适应。因此,所构建的模型正确地预测了各种化合物的跨膜渗透及其潜在的细胞内活性。
更新日期:2020-02-10
中文翻译:

两种转运蛋白对外排的药物渗透。
抗革兰氏阴性细菌的新抗生素的开发受到其细胞膜强大的保护特性的阻碍。该包膜由两个由外排转运蛋白增强的膜组成,外排转运蛋白协同作用以限制细胞接触多种化学物质。最近,已经建立了该系统的动力学模型。该模型揭示了系统的复杂,非线性行为,具有分叉,并且与实验吸收数据非常吻合。在这里,我们将模型扩展为包括多个转运蛋白,并将其应用于野生型和外排缺陷型大肠杆菌中抗生素积累的实验分析。我们表明,跨内膜和外膜起作用的转运蛋白彼此具有协同作用。相反,跨同一膜起作用的转运蛋白通常是加性的,但由于势垒常数控制的分叉,在特殊情况下可能具有协同作用。关于溴化乙锭,内膜转运蛋白MdfA与穿过外膜的TolC依赖性外排协同作用。在一系列测试药物浓度和菌株中,模型与药物积累之间的一致性非常好。但是,抗生素敏感性仅在质量上与药物的积累或模型的预测有关,并且只有在对延长的细胞长期暴露于药物的生理后果做出其他假设的情况下,抗生素敏感性才可能与模型相适应。因此,所构建的模型正确地预测了各种化合物的跨膜渗透及其潜在的细胞内活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号