当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H-Abstraction from Dimethyl Sulfide in the Presence of an Excess of Hydroxyl Radicals. A Quantum Chemical Evaluation of Thermochemical and Kinetic Parameters Unveils an Alternative Pathway to Dimethyl Sulfoxide
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-03-06 , DOI: 10.1021/acsearthspacechem.9b00306 Zoi Salta 1 , Jacopo Lupi 1 , Vincenzo Barone 1 , Oscar N. Ventura 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-03-06 , DOI: 10.1021/acsearthspacechem.9b00306 Zoi Salta 1 , Jacopo Lupi 1 , Vincenzo Barone 1 , Oscar N. Ventura 2
Affiliation
|
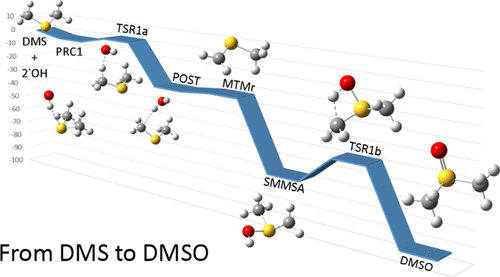
Elucidation of the oxidation mechanism of naturally emitted reduced sulfur compounds, especially dimethyl sulfide, plays a central role in understanding background acid precipitation in the natural environment. Most frequently, theoretical studies of the addition and H-elimination reactions of dimethyl sulfide with hydroxyl radicals are studied considering the presence of oxygen that further reacts with the radicals formed in the initial steps. Although the reaction of intermediate species with additional hydroxyl radicals has been considered as part of the global mechanism of oxidation, little if any attention has been dedicated to the possibility of reactions of the initial radicals with a second •OH molecule. In this work, we performed a computational study using quantum-chemical methods, of the mechanism of H-abstraction from dimethyl sulfide under normal atmospheric conditions and in reaction chambers at different O2 partial pressure, including complete absence of oxygen. Additionally, important rate coefficients were computed using canonical and variational transition state theory. The rate coefficient for abstraction affords a 4.72 × 10–12 cm3 molecule–1 s–1 value, very close to the most recent experimental one (4.13 × 10–12 cm3 molecule–1 s–1). According to our best results, the initial methyl thiomethyl radical was obtained at −25.2 kcal/mol (experimentally −22.4 kcal/mol), and four important paths were identified on the potential energy surface. From the interplay of thermochemical and kinetic arguments, it was possible to demonstrate that the preferred product of the reaction of dimethyl sulfide with two hydroxyl radicals is actually dimethyl sulfoxide.
中文翻译:

在过量的羟基自由基存在下从二甲基硫中提取H。热化学和动力学参数的量子化学评估揭示了二甲基亚砜的替代途径
阐明自然排放的还原硫化合物(尤其是二甲基硫醚)的氧化机理,对于理解自然环境中的背景酸沉淀起着核心作用。最经常地,考虑到氧的存在进一步与在初始步骤中形成的自由基反应,研究了二甲基硫与羟基自由基的加成和消除反应的理论研究。虽然额外的羟基自由基中间产物的反应一直被视为氧化的全球机制的一部分,几乎没有任何的关注一直致力于与第二初始自由基反应的可能性•OH分子。在这项工作中,我们使用量子化学方法进行了计算研究,研究了在正常大气条件下以及在不同O 2分压(包括完全没有氧气)的反应室中H从二甲基硫中吸氢的机理。此外,使用规范和变迁过渡状态理论计算了重要的速率系数。抽象的速率系数提供了4.72×10 –12 cm 3分子–1 s –1的值,非常接近最新的实验值(4.13×10 –12 cm 3分子–1 s –1)。根据我们的最佳结果,获得的初始甲硫基甲基自由基为-25.2 kcal / mol(实验值为-22.4 kcal / mol),并且在势能面上确定了四个重要路径。从热化学和动力学参数的相互作用,可以证明二甲基硫醚与两个羟基自由基反应的优选产物实际上是二甲基亚砜。
更新日期:2020-03-06
中文翻译:

在过量的羟基自由基存在下从二甲基硫中提取H。热化学和动力学参数的量子化学评估揭示了二甲基亚砜的替代途径
阐明自然排放的还原硫化合物(尤其是二甲基硫醚)的氧化机理,对于理解自然环境中的背景酸沉淀起着核心作用。最经常地,考虑到氧的存在进一步与在初始步骤中形成的自由基反应,研究了二甲基硫与羟基自由基的加成和消除反应的理论研究。虽然额外的羟基自由基中间产物的反应一直被视为氧化的全球机制的一部分,几乎没有任何的关注一直致力于与第二初始自由基反应的可能性•OH分子。在这项工作中,我们使用量子化学方法进行了计算研究,研究了在正常大气条件下以及在不同O 2分压(包括完全没有氧气)的反应室中H从二甲基硫中吸氢的机理。此外,使用规范和变迁过渡状态理论计算了重要的速率系数。抽象的速率系数提供了4.72×10 –12 cm 3分子–1 s –1的值,非常接近最新的实验值(4.13×10 –12 cm 3分子–1 s –1)。根据我们的最佳结果,获得的初始甲硫基甲基自由基为-25.2 kcal / mol(实验值为-22.4 kcal / mol),并且在势能面上确定了四个重要路径。从热化学和动力学参数的相互作用,可以证明二甲基硫醚与两个羟基自由基反应的优选产物实际上是二甲基亚砜。











































 京公网安备 11010802027423号
京公网安备 11010802027423号