当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Increased miR-34c mediates synaptic deficits by targeting synaptotagmin 1 through ROS-JNK-p53 pathway in Alzheimer's Disease.
Aging Cell ( IF 8.0 ) Pub Date : 2020-02-24 , DOI: 10.1111/acel.13125 Zhongli Shi 1, 2 , Kaixia Zhang 1 , Huimin Zhou 2 , Lei Jiang 1, 2 , Bing Xie 1 , Ruiyuan Wang 1 , Wenzhen Xia 1 , Yajuan Yin 1 , Zhaoyu Gao 1 , Dongsheng Cui 1 , Rui Zhang 1, 2 , Shunjiang Xu 1, 2
Aging Cell ( IF 8.0 ) Pub Date : 2020-02-24 , DOI: 10.1111/acel.13125 Zhongli Shi 1, 2 , Kaixia Zhang 1 , Huimin Zhou 2 , Lei Jiang 1, 2 , Bing Xie 1 , Ruiyuan Wang 1 , Wenzhen Xia 1 , Yajuan Yin 1 , Zhaoyu Gao 1 , Dongsheng Cui 1 , Rui Zhang 1, 2 , Shunjiang Xu 1, 2
Affiliation
|
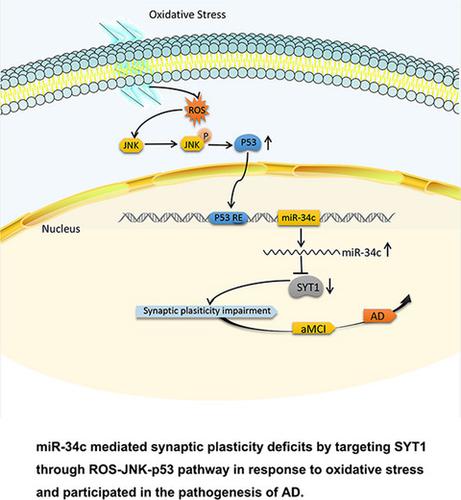
Alzheimer's disease (AD) and cancer have inverse relationship in many aspects. Some tumor suppressors, including miR‐34c, are decreased in cancer but increased in AD. The upstream regulatory pathways and the downstream mechanisms of miR‐34c in AD remain to be investigated. The expression of miR‐34c was detected by RT–qPCR in oxidative stressed neurons, hippocampus of SAMP8 mice, or serum of patients with amnestic mild cognitive impairment (aMCI). Dual luciferase assay was performed to confirm the binding sites of miR‐34c in its target mRNA. The Morris water maze (MWM) was used to evaluate learning and memory in SAMP8 mice administrated with miR‐34c antagomir (AM34c). Golgi staining was used to evaluate the synaptic function and structure. The dramatically increased miR‐34c was mediated by ROS‐JNK‐p53 pathway and negatively regulated synaptotagmin 1 (SYT1) expression by targeting the 3′‐untranslated region (3′‐UTR) of syt1 in AD. The expression of SYT1 protein was reduced by over expression of miR‐34c in the HT‐22 cells and vice versa. Administration of AM34c by the third ventricle injection or intranasal delivery markedly increased the brain levels of SYT1 and ameliorated the cognitive function in SAMP8 mice. The serum miR‐34c was significantly increased in patients with aMCI and might be a predictive biomarker for diagnosis of aMCI. These results indicated that increased miR‐34c mediated synaptic and memory deficits by targeting SYT1 through ROS‐JNK‐p53 pathway and the miR‐34c/SYT1 pathway could be considered as a promising novel therapeutic target for patients with AD.
中文翻译:

在阿尔茨海默病中,miR-34c 的增加通过 ROS-JNK-p53 通路靶向突触结合蛋白 1,从而介导突触缺陷。
阿尔茨海默病(AD)和癌症在很多方面都存在反比关系。一些肿瘤抑制因子,包括 miR-34c,在癌症中减少,但在 AD 中增加。miR-34c在AD中的上游调控途径和下游机制仍有待研究。通过 RT-qPCR 检测氧化应激神经元、SAMP8 小鼠海马或遗忘性轻度认知障碍 (aMCI) 患者血清中 miR-34c 的表达。进行双荧光素酶测定以确认 miR-34c 在其靶 mRNA 中的结合位点。Morris 水迷宫 (MWM) 用于评估给予 miR-34c antagomir (AM34c) 的 SAMP8 小鼠的学习和记忆能力。高尔基染色用于评估突触功能和结构。AD 中 ROS-JNK-p53 通路介导 miR-34c 显着增加,并通过靶向 syt1 的 3'-非翻译区 (3'-UTR) 负调节突触结合蛋白 1 (SYT1) 表达。HT-22 细胞中 miR-34c 的过表达会降低 SYT1 蛋白的表达,反之亦然。通过第三脑室注射或鼻内给药AM34c显着增加了SAMP8小鼠大脑中SYT1的水平并改善了认知功能。aMCI 患者血清 miR-34c 显着升高,可能是诊断 aMCI 的预测生物标志物。这些结果表明,通过 ROS-JNK-p53 通路靶向 SYT1 来增加 miR-34c 介导的突触和记忆缺陷,而 miR-34c/SYT1 通路可被视为 AD 患者有前途的新型治疗靶点。
更新日期:2020-02-24
中文翻译:

在阿尔茨海默病中,miR-34c 的增加通过 ROS-JNK-p53 通路靶向突触结合蛋白 1,从而介导突触缺陷。
阿尔茨海默病(AD)和癌症在很多方面都存在反比关系。一些肿瘤抑制因子,包括 miR-34c,在癌症中减少,但在 AD 中增加。miR-34c在AD中的上游调控途径和下游机制仍有待研究。通过 RT-qPCR 检测氧化应激神经元、SAMP8 小鼠海马或遗忘性轻度认知障碍 (aMCI) 患者血清中 miR-34c 的表达。进行双荧光素酶测定以确认 miR-34c 在其靶 mRNA 中的结合位点。Morris 水迷宫 (MWM) 用于评估给予 miR-34c antagomir (AM34c) 的 SAMP8 小鼠的学习和记忆能力。高尔基染色用于评估突触功能和结构。AD 中 ROS-JNK-p53 通路介导 miR-34c 显着增加,并通过靶向 syt1 的 3'-非翻译区 (3'-UTR) 负调节突触结合蛋白 1 (SYT1) 表达。HT-22 细胞中 miR-34c 的过表达会降低 SYT1 蛋白的表达,反之亦然。通过第三脑室注射或鼻内给药AM34c显着增加了SAMP8小鼠大脑中SYT1的水平并改善了认知功能。aMCI 患者血清 miR-34c 显着升高,可能是诊断 aMCI 的预测生物标志物。这些结果表明,通过 ROS-JNK-p53 通路靶向 SYT1 来增加 miR-34c 介导的突触和记忆缺陷,而 miR-34c/SYT1 通路可被视为 AD 患者有前途的新型治疗靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号