当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability Series for the Complexation of Six Key Siderophore Functional Groups with Uranyl Using Density Functional Theory
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.jpca.9b10649 Matthew Edward Kirby 1 , Jason Louis Sonnenberg 2 , Alexandra Simperler 3 , Dominik Jakob Weiss 1, 4
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-16 , DOI: 10.1021/acs.jpca.9b10649 Matthew Edward Kirby 1 , Jason Louis Sonnenberg 2 , Alexandra Simperler 3 , Dominik Jakob Weiss 1, 4
Affiliation
|
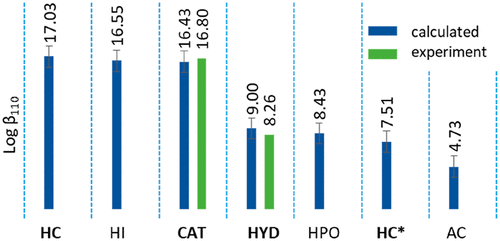
Determining stability constants of uranyl complexes with the principal functional groups in siderophores and identifying stability series is of great importance to predict which siderophore classes preferentially bind to UVI and, hence, impact uranium speciation in the environment. It also helps to develop resins for scavenging UVI from aqueous solutions. Here, we apply a recently developed computational approach to calculate log β values for a set of geochemically relevant uranium organometallic complexes using Density Functional Theory (DFT). We determined the stability series for catecholate, hydroxamate, α-hydroxycarboxylate, α-aminocarboxylate, hydroxy-phenyloxazolonate, and α-hydroxyimidazole with the uranyl cation. In this work, the stability constants (log β110) of α-hydroxyimidazolate and hydroxy-phenyloxazolonate are calculated for the first time. Our approach employed the B3LYP density functional approximation, aug-cc-pVDZ basis set for ligand atoms, MDF60 ECP for UVI, and the IEFPCM solvation model. DFT calculated log β110 were corrected using a previously established fitting equation. We find that the siderophore functional groups stability decreases in the order: α-hydroxycarboxylate bound via the α-hydroxy and carboxylate groups (log β110 = 17.08), α-hydroxyimidazolate (log β110 = 16.55), catecholate (log β110 = 16.43), hydroxamate (log β110 = 9.00), hydroxy-phenyloxazolonate (log β110 = 8.43), α-hydroxycarboxylate bound via the carboxylate group (log β110 = 7.51) and α-aminocarboxylate (log β110 = 4.73). We confirm that the stability for the binding mode of the functional groups decrease in the order: bidentate, monodentate via ligand O atoms, and monodentate via ligand N atoms. The stability series strongly suggests that α-hydroxyimidazolate is an important functional group that needs to be included when assessing uranyl mobility and removal from aqueous solutions.
中文翻译:

密度泛函理论的六个关键铁载体官能团与铀酰络合的稳定性系列
确定铁载体中具有主要官能团的铀酰配合物的稳定常数并确定稳定性系列,对于预测哪些铁载体类优先结合U VI并因此影响环境中的铀形态非常重要。它还有助于开发用于从水溶液中清除U VI的树脂。在这里,我们采用最新开发的计算方法,使用密度泛函理论(DFT)为一组地球化学相关的铀有机金属配合物计算logβ值。我们确定了邻苯二甲酸酯,异羟肟酸酯,α-羟基羧酸酯,α-氨基羧酸酯,羟基-苯恶唑酮酸酯和α-羟基咪唑与铀酰阳离子的稳定性系列。在这项工作中,稳定常数(日志β 110首次计算α)-羟基咪唑啉酸酯和羟基-苯基恶唑酮酸酯。我们的方法采用B3LYP密度泛函近似,配体原子的aug-cc-pVDZ基集,U VI的MDF60 ECP和IEFPCM溶剂化模型。DFT计算日志β 110使用先前建立的拟合方程校正。我们发现,铁载体的官能团的稳定性的顺序降低:通过α羟基和羧酸酯基团结合的α羟基羧酸(日志β 110 = 17.08),α-hydroxyimidazolate(日志β 110 = 16.55),儿茶酚(日志β 110 = 16.43),异羟肟酸(日志β 110 = 9.00),羟基- phenyloxazolonate(日志β 110= 8.43),α羟基羧酸通过羧酸基团结合的(日志β 110 = 7.51)和α-氨基羧酸盐(日志β 110 = 4.73)。我们确认,官能团结合模式的稳定性依次降低:二齿,通过配体O原子单齿和通过配体N原子单齿。稳定性系列强烈表明,在评估铀酰迁移率和从水溶液中去除时,α-羟基咪唑酸酯是重要的官能团。
更新日期:2020-03-16
中文翻译:

密度泛函理论的六个关键铁载体官能团与铀酰络合的稳定性系列
确定铁载体中具有主要官能团的铀酰配合物的稳定常数并确定稳定性系列,对于预测哪些铁载体类优先结合U VI并因此影响环境中的铀形态非常重要。它还有助于开发用于从水溶液中清除U VI的树脂。在这里,我们采用最新开发的计算方法,使用密度泛函理论(DFT)为一组地球化学相关的铀有机金属配合物计算logβ值。我们确定了邻苯二甲酸酯,异羟肟酸酯,α-羟基羧酸酯,α-氨基羧酸酯,羟基-苯恶唑酮酸酯和α-羟基咪唑与铀酰阳离子的稳定性系列。在这项工作中,稳定常数(日志β 110首次计算α)-羟基咪唑啉酸酯和羟基-苯基恶唑酮酸酯。我们的方法采用B3LYP密度泛函近似,配体原子的aug-cc-pVDZ基集,U VI的MDF60 ECP和IEFPCM溶剂化模型。DFT计算日志β 110使用先前建立的拟合方程校正。我们发现,铁载体的官能团的稳定性的顺序降低:通过α羟基和羧酸酯基团结合的α羟基羧酸(日志β 110 = 17.08),α-hydroxyimidazolate(日志β 110 = 16.55),儿茶酚(日志β 110 = 16.43),异羟肟酸(日志β 110 = 9.00),羟基- phenyloxazolonate(日志β 110= 8.43),α羟基羧酸通过羧酸基团结合的(日志β 110 = 7.51)和α-氨基羧酸盐(日志β 110 = 4.73)。我们确认,官能团结合模式的稳定性依次降低:二齿,通过配体O原子单齿和通过配体N原子单齿。稳定性系列强烈表明,在评估铀酰迁移率和从水溶液中去除时,α-羟基咪唑酸酯是重要的官能团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号