Cell Calcium ( IF 4.3 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.ceca.2020.102184 Lukun Yang 1 , Alla F Fomina 2
|
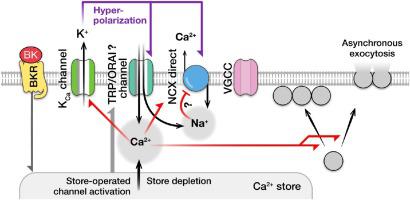
Neuroendocrine adrenal chromaffin cells release neurohormones catecholamines in response to Ca2+ entry via voltage-gated Ca2+ channels (VGCCs). Adrenal chromaffin cells also express non-voltage-gated channels, which may conduct Ca2+ at negative membrane potentials, whose role in regulation of exocytosis is poorly understood. We explored how modulation of Ca2+ influx at negative membrane potentials affects basal cytosolic Ca2+ concentration ([Ca2+]i) and exocytosis in metabolically intact voltage-clamped bovine adrenal chromaffin cells. We found that in these cells, Ca2+ entry at negative membrane potentials is balanced by Ca2+ extrusion by the Na+/Ca2+ exchanger and that this balance can be altered by membrane hyperpolarization or stimulation with an inflammatory hormone bradykinin. Membrane hyperpolarization or application of bradykinin augmented Ca2+-carrying current at negative membrane potentials, elevated basal [Ca2+]i, and facilitated synchronous exocytosis evoked by the small amounts of Ca2+ injected into the cell via VGCCs (up to 20 pC). Exocytotic responses evoked by the injections of the larger amounts of Ca2+ via VGCCs (> 20 pC) were suppressed by preceding hyperpolarization. In the absence of Ca2+ entry via VGCCs and Ca2+ extrusion via the Na+/Ca2+ exchanger, membrane hyperpolarization induced a significant elevation in [Ca2+]i and asynchronous exocytosis. Our results indicate that physiological interferences, such as membrane hyperpolarization and/or activation of non-voltage-gated Ca2+ channels, modulate basal [Ca2+]i and, consequently, segregation of exocytotic vesicles and their readiness to be released spontaneously and in response to Ca2+ entry via VGCCs. These mechanisms may play role in homeostatic plasticity of neuronal and endocrine cells.
中文翻译:

Ca2 +的流入和清除在超极化膜电位上调节神经内分泌细胞的自发性和刺激性胞吐作用。
神经内分泌肾上腺嗜铬细胞通过电压门控的Ca 2+通道(VGCC)响应Ca 2+的进入而释放神经激素儿茶酚胺。肾上腺嗜铬细胞还表达非电压门控通道,其可能在负膜电位下传导Ca 2+,其在胞吐作用调节中的作用了解甚少。我们探讨了在负膜电位处Ca 2+内流的调节如何影响代谢完整的电压钳制牛肾上腺嗜铬细胞中的基础胞质Ca 2+浓度([Ca 2+ ] i)和胞吐作用。我们发现在这些细胞中,Ca 2+在负膜电位处的进入被Ca平衡通过Na + / Ca 2+交换剂进行2+挤出,这种平衡可以通过膜超极化或炎性激素缓激肽的刺激来改变。膜超极化或缓激肽的应用在负膜电位下增加了Ca 2+的载流电流,增加了基础[Ca 2+ ] i,并促进了通过VGCC注入细胞中的少量Ca 2+引起的同步胞吐作用pC)。通过VGCC(> 20 pC)注入大量Ca 2+引起的胞吐反应被先前的超极化抑制了。在没有Ca 2+通过VGCC和Ca进入的情况下通过Na + / Ca 2+交换剂2+挤出,膜超极化诱导[Ca 2+ ] i显着升高和异步胞吐作用。我们的结果表明,生理干扰,例如膜超极化和/或非电压门控的Ca 2+通道的激活,可调节基础[Ca 2+ ] i,因此,胞吐小泡的分离及其易于自发释放和响应通过VGCC进入Ca 2+。这些机制可能在神经元和内分泌细胞的稳态可塑性中起作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号