Nature Medicine ( IF 58.7 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41591-020-0763-1 Jasmina Cehajic-Kapetanovic 1, 2 , Kanmin Xue 1, 2 , Cristina Martinez-Fernandez de la Camara 1, 2 , Anika Nanda 1, 2 , Alexandra Davies 1, 2 , Laura J Wood 1, 2 , Anna Paola Salvetti 1, 2 , M Dominik Fischer 1 , James W Aylward 1, 2 , Alun R Barnard 1, 2 , Jasleen K Jolly 1, 2 , Edmond Luo 3 , Brandon J Lujan 4 , Tuyen Ong 3 , Aniz Girach 3 , Graeme C M Black 5, 6 , Ninel Z Gregori 7 , Janet L Davis 7 , Potyra R Rosa 7 , Andrew J Lotery 8, 9 , Byron L Lam 7 , Paulo E Stanga 5, 6 , Robert E MacLaren 1, 2
|
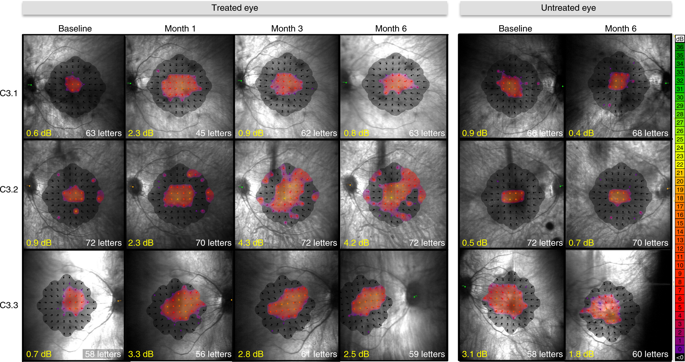
Retinal gene therapy has shown great promise in treating retinitis pigmentosa (RP), a primary photoreceptor degeneration that leads to severe sight loss in young people. In the present study, we report the first-in-human phase 1/2, dose-escalation clinical trial for X-linked RP caused by mutations in the RP GTPase regulator (RPGR) gene in 18 patients over up to 6 months of follow-up (https://clinicaltrials.gov/: NCT03116113). The primary outcome of the study was safety, and secondary outcomes included visual acuity, microperimetry and central retinal thickness. Apart from steroid-responsive subretinal inflammation in patients at the higher doses, there were no notable safety concerns after subretinal delivery of an adeno-associated viral vector encoding codon-optimized human RPGR (AAV8-coRPGR), meeting the pre-specified primary endpoint. Visual field improvements beginning at 1 month and maintained to the last point of follow-up were observed in six patients.
中文翻译:

针对 RPGR 突变引起的 X 连锁色素性视网膜炎的首次人体基因治疗试验的初步结果
视网膜基因疗法在治疗色素性视网膜炎(RP)方面显示出巨大的前景,这是一种原发性光感受器变性,会导致年轻人严重失明。在本研究中,我们报告了首个人体 1/2 期剂量递增临床试验,针对由 RP GTPase 调节因子 ( RPGR )基因突变引起的 X 连锁 RP ,在 18 名患者中进行了长达 6 个月的随访-up(https://clinicaltrials.gov/:NCT03116113)。该研究的主要结果是安全性,次要结果包括视力、微视野检查和中央视网膜厚度。除了较高剂量下患者出现类固醇反应性视网膜下炎症外,视网膜下递送编码密码子优化的人RPGR (AAV8 -coRPGR ) 的腺相关病毒载体后,没有出现明显的安全问题,达到了预先指定的主要终点。在 6 名患者中观察到视野从 1 个月开始改善并维持到随访的最后一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号