当前位置:
X-MOL 学术
›
Part. Part. Syst. Charact.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal–Drug–Protein Assemblies: Gd3+ Self‐Enhanced Magnetic Resonance Imaging, High‐Sensitive Tumor‐Targeting Imaging and Efficient Chemo‐Phototherapy
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.1002/ppsc.201900427 Jia Zhou 1 , Tianliang Li 2 , Yiran Zhang 1 , Xiaoyu Xu 1 , Chunlei Zhang 2 , Yuehua Li 1 , Daxiang Cui 2 , Yingsheng Cheng 1
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.1002/ppsc.201900427 Jia Zhou 1 , Tianliang Li 2 , Yiran Zhang 1 , Xiaoyu Xu 1 , Chunlei Zhang 2 , Yuehua Li 1 , Daxiang Cui 2 , Yingsheng Cheng 1
Affiliation
|
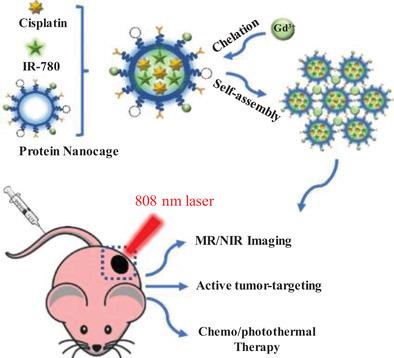
Magnetic resonance imaging (MRI) contrast agents are broadly employed for better clinical trials in MR imaging. Magnevist solution (Gd‐DTPA), a clinical MRI contrast agent, possesses inherent shortcomings like poor r1 relaxation, short half‐time, nephrotoxicity, etc. To overcome these problems, Gd‐DTPA‐grafted protein assemblies (Gd‐P‐ABs) loading with anticancer drug cisplatin and photosentizer IR‐780 are constructed via chelation of Gd3+. Gd‐P‐ABs exhibit dual MR/fluorescence (FL) imaging–guided chemo/photothermal therapy. Interestingly, Gd‐P‐ABs behave as aggregation‐enhanced magnetic resonance imaging with an extremely high r1 value of 26.391 s−1 mm−1, which is about 5.5‐fold larger than Gd‐DTPA (≈4.8 s−1 mm−1). Consequently, better MRI performance is presented with the same concentration of Gd ions. When exposed to acidic tumor microenvironment and light irradiation, Gd‐P‐ABs show significant drug release capacity. Good cell killing ability in vitro is also determined due to effective folate‐targeting ability and high photo–heat conversion. In vivo MR/FL imaging results reveal that Gd‐P‐ABs possess high‐sensitivity tumor‐targeting imaging and long tumor retention, which are attributed to the folate‐targeting ability and small size effect. Combined chemo/photothermal therapy in vivo demonstrates that the tumor can be eventually ablated. Altogether, the Gd‐P‐ABs possess great potential for clinical imaging‐guided tumor therapy.
中文翻译:

金属-药物-蛋白质组件:Gd3 +自增强磁共振成像,高灵敏度肿瘤靶向成像和高效化学光疗
磁共振成像(MRI)造影剂被广泛用于MR成像的更好的临床试验。Magnevist解决方案(Gd-DTPA)是一种临床MRI造影剂,具有固有的缺点,如r 1松弛差,半衰期短,肾毒性等。为克服这些问题,可将Gd-DTPA移植的蛋白质组装体(Gd-P-ABs )通过Gd 3+的螯合来构建抗癌药顺铂和光敏剂IR‐780的负载。Gd-P-AB具有双重MR /荧光(FL)成像引导的化学/光热疗法。有趣的是,Gd-P-AB表现为聚集增强的磁共振成像,其r 1值为26.391 s -1 m m -1极高约为Gd-DTPA的5.5倍(≈4.8s -1 m m -1)。因此,在相同浓度的Gd离子下,MRI表现更好。当暴露于酸性肿瘤微环境和光照下时,Gd-P-ABs表现出显着的药物释放能力。由于有效的叶酸靶向能力和高的光热转化率,在体外也具有良好的细胞杀伤能力。体内MR / FL成像结果表明,Gd-P-AB具有高敏感性的肿瘤靶向成像和长的肿瘤保留期,这归因于叶酸靶向能力和小尺寸效应。体内化学/光热疗法相结合证明了肿瘤最终可以消融。总之,Gd-P-AB具有巨大的潜力,可用于临床影像引导的肿瘤治疗。
更新日期:2020-01-31
中文翻译:

金属-药物-蛋白质组件:Gd3 +自增强磁共振成像,高灵敏度肿瘤靶向成像和高效化学光疗
磁共振成像(MRI)造影剂被广泛用于MR成像的更好的临床试验。Magnevist解决方案(Gd-DTPA)是一种临床MRI造影剂,具有固有的缺点,如r 1松弛差,半衰期短,肾毒性等。为克服这些问题,可将Gd-DTPA移植的蛋白质组装体(Gd-P-ABs )通过Gd 3+的螯合来构建抗癌药顺铂和光敏剂IR‐780的负载。Gd-P-AB具有双重MR /荧光(FL)成像引导的化学/光热疗法。有趣的是,Gd-P-AB表现为聚集增强的磁共振成像,其r 1值为26.391 s -1 m m -1极高约为Gd-DTPA的5.5倍(≈4.8s -1 m m -1)。因此,在相同浓度的Gd离子下,MRI表现更好。当暴露于酸性肿瘤微环境和光照下时,Gd-P-ABs表现出显着的药物释放能力。由于有效的叶酸靶向能力和高的光热转化率,在体外也具有良好的细胞杀伤能力。体内MR / FL成像结果表明,Gd-P-AB具有高敏感性的肿瘤靶向成像和长的肿瘤保留期,这归因于叶酸靶向能力和小尺寸效应。体内化学/光热疗法相结合证明了肿瘤最终可以消融。总之,Gd-P-AB具有巨大的潜力,可用于临床影像引导的肿瘤治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号