当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of enzymatic reduction on bivalent bispecific antibody fragmentation and loss of product purity upon reoxidation.
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-01-12 , DOI: 10.1002/bit.27264 Nicole Swope 1 , Wai Keen Chung 2 , Mingyan Cao 3 , Dana Motabar 2 , Dengfeng Liu 3 , Sanjeev Ahuja 4 , Michael Handlogten 4
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-01-12 , DOI: 10.1002/bit.27264 Nicole Swope 1 , Wai Keen Chung 2 , Mingyan Cao 3 , Dana Motabar 2 , Dengfeng Liu 3 , Sanjeev Ahuja 4 , Michael Handlogten 4
Affiliation
|
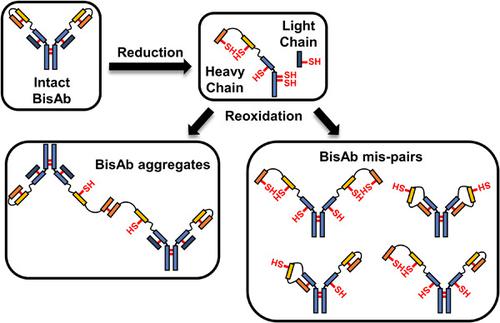
Antibody disulfide bond (DSB) reduction during manufacturing processes is a widely observed phenomenon attributed to host cell reductases present in harvest cell culture fluid. Enzyme-induced antibody reduction leads to product fragments and aggregates that increase the impurity burden on the purification process. The impact of reduction on bivalent bispecific antibodies (BisAbs), which are increasingly entering the clinic, has yet to be investigated. We focused on the reduction and reoxidation properties of a homologous library of bivalent BisAb formats that possess additional single-chain Fv (scFv) fragments with engineered DSBs. Despite all BisAbs having similar susceptibilities to enzymatic reduction, fragmentation pathways were dependent on the scFv-fusion site. Reduced molecules were allowed to reoxidize with and without low pH viral inactivation treatment. Both reoxidation studies demonstrated that multiple, complex BisAb species formed as a result of DSB mispairing. Furthermore, aggregate levels increased for all molecules when no low pH treatment was applied. Combined, our results show that complex DSB mispairing occurs during downstream processes while aggregate formation is dependent on sample treatment. These results are applicable to other novel monoclonal antibody-like formats containing engineered DSBs, thus highlighting the need to prevent reduction of novel protein therapeutics to avoid diminished product quality during manufacturing.
中文翻译:

酶还原对二价双特异性抗体片段化和再氧化时产品纯度损失的影响。
制造过程中抗体二硫键 (DSB) 的还原是一种广泛观察到的现象,归因于收获细胞培养液中存在的宿主细胞还原酶。酶诱导的抗体还原会导致产物碎片和聚集体,从而增加纯化过程中的杂质负担。减少对越来越多地进入临床的二价双特异性抗体(BisAb)的影响还有待研究。我们重点研究二价 BisAb 形式同源文库的还原和再氧化特性,该同源文库拥有带有工程化 DSB 的额外单链 Fv (scFv) 片段。尽管所有 BisAb 对酶促还原具有相似的敏感性,但片段化途径依赖于 scFv 融合位点。在有或没有低pH值病毒灭活处理的情况下,还原分子都可以重新氧化。两项再氧化研究均表明,由于 DSB 错配而形成了多种复杂的 BisAb 物种。此外,当不进行低 pH 处理时,所有分子的聚集体水平都会增加。综合起来,我们的结果表明,复杂的 DSB 错配发生在下游过程中,而聚集体的形成取决于样品处理。这些结果适用于其他含有工程 DSB 的新型单克隆抗体样形式,因此强调需要防止新型蛋白质治疗药物的减少,以避免在制造过程中产品质量下降。
更新日期:2020-03-09
中文翻译:

酶还原对二价双特异性抗体片段化和再氧化时产品纯度损失的影响。
制造过程中抗体二硫键 (DSB) 的还原是一种广泛观察到的现象,归因于收获细胞培养液中存在的宿主细胞还原酶。酶诱导的抗体还原会导致产物碎片和聚集体,从而增加纯化过程中的杂质负担。减少对越来越多地进入临床的二价双特异性抗体(BisAb)的影响还有待研究。我们重点研究二价 BisAb 形式同源文库的还原和再氧化特性,该同源文库拥有带有工程化 DSB 的额外单链 Fv (scFv) 片段。尽管所有 BisAb 对酶促还原具有相似的敏感性,但片段化途径依赖于 scFv 融合位点。在有或没有低pH值病毒灭活处理的情况下,还原分子都可以重新氧化。两项再氧化研究均表明,由于 DSB 错配而形成了多种复杂的 BisAb 物种。此外,当不进行低 pH 处理时,所有分子的聚集体水平都会增加。综合起来,我们的结果表明,复杂的 DSB 错配发生在下游过程中,而聚集体的形成取决于样品处理。这些结果适用于其他含有工程 DSB 的新型单克隆抗体样形式,因此强调需要防止新型蛋白质治疗药物的减少,以避免在制造过程中产品质量下降。











































 京公网安备 11010802027423号
京公网安备 11010802027423号