当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β2-adrenergic Agonists Rescue Lysosome Acidification and Function in PSEN1 Deficiency by Reversing Defective ER-to-lysosome Delivery of ClC-7.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.jmb.2020.02.021 Ju-Hyun Lee 1 , Devin M Wolfe 2 , Sandipkumar Darji 2 , Mary Kate McBrayer 2 , Daniel J Colacurcio 2 , Asok Kumar 2 , Philip Stavrides 2 , Panaiyur S Mohan 1 , Ralph A Nixon 3
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.jmb.2020.02.021 Ju-Hyun Lee 1 , Devin M Wolfe 2 , Sandipkumar Darji 2 , Mary Kate McBrayer 2 , Daniel J Colacurcio 2 , Asok Kumar 2 , Philip Stavrides 2 , Panaiyur S Mohan 1 , Ralph A Nixon 3
Affiliation
|
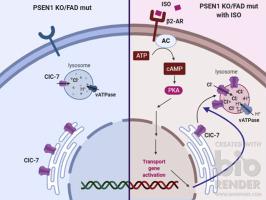
Lysosomal dysfunction is considered pathogenic in Alzheimer disease (AD). Loss of presenilin-1 (PSEN1) function causing AD impedes acidification via defective vacuolar ATPase (vATPase) V0a1 subunit delivery to lysosomes. We report that isoproterenol (ISO) and related β2-adrenergic agonists reacidify lysosomes in PSEN1 Knock out (KO) cells and fibroblasts from PSEN1 familial AD patients, which restores lysosomal proteolysis, calcium homeostasis, and normal autophagy flux. We identify a novel rescue mechanism involving Portein Kinase A (PKA)-mediated facilitation of chloride channel-7 (ClC-7) delivery to lysosomes which reverses markedly lowered chloride (Cl-) content in PSEN1 KO lysosomes. Notably, PSEN1 loss of function impedes Endoplasmic Reticulum (ER)-to-lysosome delivery of ClC-7. Transcriptomics of PSEN1-deficient cells reveals strongly downregulated ER-to-lysosome transport pathways and reversibility by ISO, thus accounting for lysosomal Cl- deficits that compound pH elevation due to deficient vATPase and its rescue by β2-adrenergic agonists. Our findings uncover a broadened PSEN1 role in lysosomal ion homeostasis and novel pH modulation of lysosomes through β2-adrenergic regulation of ClC-7, which can potentially be modulated therapeutically.
中文翻译:

β2-肾上腺素能激动剂通过逆转ClC-7的ER到溶酶体的有缺陷传递来挽救PSEN1缺陷的溶酶体酸化和功能。
溶酶体功能障碍被认为是阿尔茨海默病(AD)的致病性。导致AD的早老素1(PSEN1)功能丧失会通过液泡ATPase(vATPase)V0a1亚基向溶酶体的传递而阻碍酸化。我们报告说,异丙肾上腺素(ISO)和相关的β2-肾上腺素能激动剂使PSEN1家族性AD患者的PSEN1敲除(KO)细胞和成纤维细胞中的溶酶体重新酸化,从而恢复了溶酶体蛋白水解,钙稳态和正常的自噬通量。我们确定了一种新型的救援机制,涉及门蛋白酶激酶A(PKA)介导的向溶酶体传递氯离子通道7(ClC-7)的促进作用,从而逆转了PSEN1 KO溶酶体中明显降低的氯离子(Cl-)含量。值得注意的是,PSEN1功能丧失会阻止内质网(ER)向溶酶体传递ClC-7。PSEN1缺陷细胞的转录组学揭示了ER到溶酶体的运输途径被强烈下调以及ISO的可逆性,因此解释了溶酶体Cl缺陷,该缺陷导致复合物由于缺乏vATPase而导致pH升高,并被β2-肾上腺素能激动剂拯救。我们的发现揭示了PSEN1在溶酶体离子稳态中的广泛作用以及通过ClC-7的β2-肾上腺素能调节溶酶体的新型pH调节,这可能在治疗中得到调节。
更新日期:2020-02-24
中文翻译:

β2-肾上腺素能激动剂通过逆转ClC-7的ER到溶酶体的有缺陷传递来挽救PSEN1缺陷的溶酶体酸化和功能。
溶酶体功能障碍被认为是阿尔茨海默病(AD)的致病性。导致AD的早老素1(PSEN1)功能丧失会通过液泡ATPase(vATPase)V0a1亚基向溶酶体的传递而阻碍酸化。我们报告说,异丙肾上腺素(ISO)和相关的β2-肾上腺素能激动剂使PSEN1家族性AD患者的PSEN1敲除(KO)细胞和成纤维细胞中的溶酶体重新酸化,从而恢复了溶酶体蛋白水解,钙稳态和正常的自噬通量。我们确定了一种新型的救援机制,涉及门蛋白酶激酶A(PKA)介导的向溶酶体传递氯离子通道7(ClC-7)的促进作用,从而逆转了PSEN1 KO溶酶体中明显降低的氯离子(Cl-)含量。值得注意的是,PSEN1功能丧失会阻止内质网(ER)向溶酶体传递ClC-7。PSEN1缺陷细胞的转录组学揭示了ER到溶酶体的运输途径被强烈下调以及ISO的可逆性,因此解释了溶酶体Cl缺陷,该缺陷导致复合物由于缺乏vATPase而导致pH升高,并被β2-肾上腺素能激动剂拯救。我们的发现揭示了PSEN1在溶酶体离子稳态中的广泛作用以及通过ClC-7的β2-肾上腺素能调节溶酶体的新型pH调节,这可能在治疗中得到调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号