当前位置:
X-MOL 学术
›
Brain Stimul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A preliminary study of Parkinson’s gene therapy via sono-magnetic sensing gene vector for conquering extra/intracellular barriers in mice
Brain Stimulation ( IF 7.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.brs.2020.02.024 Chun-Yao Wu , Rih-Yang Huang , En-Chi Liao , Yu-Chun Lin , Yi-Ju Ho , Chien-Wen Chang , Hong-Lin Chan , Ying-Zu Huang , Tsung-Hsun Hsieh , Ching-Hsiang Fan , Chih-Kuang Yeh
Brain Stimulation ( IF 7.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.brs.2020.02.024 Chun-Yao Wu , Rih-Yang Huang , En-Chi Liao , Yu-Chun Lin , Yi-Ju Ho , Chien-Wen Chang , Hong-Lin Chan , Ying-Zu Huang , Tsung-Hsun Hsieh , Ching-Hsiang Fan , Chih-Kuang Yeh
|
BACKGROUND
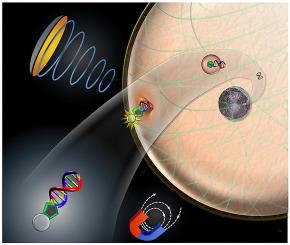
Non-virus genetic treatment for Parkinson's disease (PD) via plasmid glial cell-line derived neurotrophic factor (pGDNF) has shown potential for repairing damaged dopaminergic neurons. However, development of this gene therapy is largely hampered by the insufficient transfection efficiency as a result of the cell membrane, lysosome, and cytoskeleton meshwork. METHODS
In this study, we propose the use of polyethylenimine (PEI)-superparamagnetic iron oxide-plasmid DNA (pDNA)-loaded microbubbles (PSp-MBs) in conjunction with focused ultrasound (FUS) and two-step magnetic navigation to provide cavitation, proton sponge effect and magnetic effects to increase the efficiency of gene delivery. RESULTS
The gene transfection rate in the proposed system was 2.2-fold higher than that of the commercial agent (TransIT®-LT1). The transfection rate could be boosted ∼11%, ∼10%, and 6% by cavitation-magnetic hybrid enhanced cell membrane permeabilization, proton sponge effect, and magnetic-assisted cytoskeleton-reorganization, respectively. In vivo data suggested that effective gene delivery with this system results in a 3.2-fold increase in recovery of dopaminergic neurons and a 3.9-fold improvement in the motor behavior when compared to untreated genetic PD mice. CONCLUSIONS
We proposed that this novel FUS-magnetic hybrid gene delivery platform could be integrated with a variety of therapeutic genes for treating neurodegenerative diseases in the future.
中文翻译:

声磁传感基因载体攻克帕金森病小鼠细胞外/细胞内屏障的初步研究
背景通过质粒神经胶质细胞系衍生的神经营养因子(pGDNF)对帕金森病(PD)的非病毒遗传治疗已显示出修复受损多巴胺能神经元的潜力。然而,由于细胞膜、溶酶体和细胞骨架网络导致转染效率不足,这种基因疗法的发展在很大程度上受到阻碍。方法 在这项研究中,我们建议使用聚乙烯亚胺 (PEI)-超顺磁性氧化铁质粒 DNA (pDNA) 负载微泡 (PSp-MBs) 结合聚焦超声 (FUS) 和两步磁导航来提供空化,质子海绵效应和磁效应,以提高基因传递的效率。结果 拟议系统中的基因转染率比商业代理 (TransIT®-LT1) 高 2.2 倍。通过空化-磁杂化增强细胞膜透化、质子海绵效应和磁辅助细胞骨架重组,转染率可分别提高~11%、~10%和6%。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论 我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。
更新日期:2020-05-01
中文翻译:

声磁传感基因载体攻克帕金森病小鼠细胞外/细胞内屏障的初步研究
背景通过质粒神经胶质细胞系衍生的神经营养因子(pGDNF)对帕金森病(PD)的非病毒遗传治疗已显示出修复受损多巴胺能神经元的潜力。然而,由于细胞膜、溶酶体和细胞骨架网络导致转染效率不足,这种基因疗法的发展在很大程度上受到阻碍。方法 在这项研究中,我们建议使用聚乙烯亚胺 (PEI)-超顺磁性氧化铁质粒 DNA (pDNA) 负载微泡 (PSp-MBs) 结合聚焦超声 (FUS) 和两步磁导航来提供空化,质子海绵效应和磁效应,以提高基因传递的效率。结果 拟议系统中的基因转染率比商业代理 (TransIT®-LT1) 高 2.2 倍。通过空化-磁杂化增强细胞膜透化、质子海绵效应和磁辅助细胞骨架重组,转染率可分别提高~11%、~10%和6%。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论 我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。体内数据表明,与未治疗的遗传性 PD 小鼠相比,该系统的有效基因传递使多巴胺能神经元的恢复增加了 3.2 倍,运动行为提高了 3.9 倍。结论我们提出,这种新型的 FUS-磁性杂交基因递送平台可以与多种治疗基因整合,以在未来治疗神经退行性疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号