当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncolytic Adenovirus Armed with BiTE, Cytokine, and Checkpoint Inhibitor Enables CAR T Cells to Control the Growth of Heterogeneous Tumors.
Molecular Therapy ( IF 12.1 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.ymthe.2020.02.016 Caroline E Porter 1 , Amanda Rosewell Shaw 1 , Youngrock Jung 1 , Tiffany Yip 1 , Patricia D Castro 2 , Vlad C Sandulache 3 , Andrew Sikora 4 , Stephen Gottschalk 5 , Michael M Ittman 2 , Malcolm K Brenner 6 , Masataka Suzuki 1
Molecular Therapy ( IF 12.1 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.ymthe.2020.02.016 Caroline E Porter 1 , Amanda Rosewell Shaw 1 , Youngrock Jung 1 , Tiffany Yip 1 , Patricia D Castro 2 , Vlad C Sandulache 3 , Andrew Sikora 4 , Stephen Gottschalk 5 , Michael M Ittman 2 , Malcolm K Brenner 6 , Masataka Suzuki 1
Affiliation
|
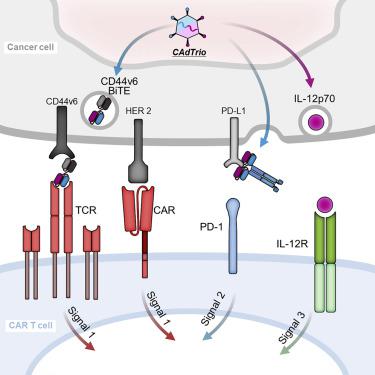
No single cancer immunotherapy will likely defeat all evasion mechanisms of solid tumors, including plasticity of tumor antigen expression and active immune suppression by the tumor environment. In this study, we increase the breadth, potency, and duration of anti-tumor activity of chimeric antigen receptor (CAR) T cells using an oncolytic virus (OV) that produces cytokine, checkpoint blockade, and a bispecific tumor-targeted T cell engager (BiTE) molecule. First, we constructed a BiTE molecule specific for CD44 variant 6 (CD44v6), since CD44v6 is widely expressed on tumor but not normal tissue, and a CD44v6 antibody has been safely administered to cancer patients. We then incorporated this BiTE sequence into an oncolytic-helper binary adenovirus (CAdDuo) encoding an immunostimulatory cytokine (interleukin [IL]-12) and an immune checkpoint blocker (PD-L1Ab) to form CAdTrio. CD44v6 BiTE from CAdTrio enabled HER2-specific CAR T cells to kill multiple CD44v6+ cancer cell lines and to produce more rapid and sustained disease control of orthotopic HER2+ and HER2-/- CD44v6+ tumors than any component alone. Thus, the combination of CAdTrio with HER2.CAR T cells ensures dual targeting of two tumor antigens by engagement of distinct classes of receptor (CAR and native T cell receptor [TCR]), and significantly improves tumor control and survival.
中文翻译:

带有BiTE,细胞因子和检查点抑制剂的溶瘤腺病毒使CAR T细胞能够控制异质性肿瘤的生长。
没有一种单一的癌症免疫疗法可能会击败实体肿瘤的所有逃避机制,包括肿瘤抗原表达的可塑性和肿瘤环境对免疫系统的积极抑制。在这项研究中,我们使用溶瘤病毒(OV)来产生嵌合因子受体(CAR)T细胞,从而产生细胞因子,检查点阻断和靶向肿瘤的双特异性T细胞接合剂,从而提高其广度,效力和抗肿瘤活性的持续时间(BiTE)分子。首先,我们构建了对CD44变体6(CD44v6)特异的BiTE分子,因为CD44v6在肿瘤上广泛表达,但在正常组织中却不表达,并且CD44v6抗体已安全地施用于癌症患者。然后,我们将此BiTE序列整合到溶瘤辅助二进制腺病毒(CAdDuo)中,以编码免疫刺激性细胞因子(白介素[IL] -12)和免疫检查点阻断剂(PD-L1Ab)形成CAdTrio。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。
更新日期:2020-02-24
中文翻译:

带有BiTE,细胞因子和检查点抑制剂的溶瘤腺病毒使CAR T细胞能够控制异质性肿瘤的生长。
没有一种单一的癌症免疫疗法可能会击败实体肿瘤的所有逃避机制,包括肿瘤抗原表达的可塑性和肿瘤环境对免疫系统的积极抑制。在这项研究中,我们使用溶瘤病毒(OV)来产生嵌合因子受体(CAR)T细胞,从而产生细胞因子,检查点阻断和靶向肿瘤的双特异性T细胞接合剂,从而提高其广度,效力和抗肿瘤活性的持续时间(BiTE)分子。首先,我们构建了对CD44变体6(CD44v6)特异的BiTE分子,因为CD44v6在肿瘤上广泛表达,但在正常组织中却不表达,并且CD44v6抗体已安全地施用于癌症患者。然后,我们将此BiTE序列整合到溶瘤辅助二进制腺病毒(CAdDuo)中,以编码免疫刺激性细胞因子(白介素[IL] -12)和免疫检查点阻断剂(PD-L1Ab)形成CAdTrio。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。来自CAdTrio的CD44v6 BiTE使HER2特异的CAR T细胞能够杀死多个CD44v6 +癌细胞系,并比单独的任何成分产生对原位HER2 +和HER2-/-CD44v6 +肿瘤的更快,更持久的疾病控制。因此,CAdTrio与HER2.CAR T细胞的结合通过不同类别的受体(CAR和天然T细胞受体[TCR])的结合,确保了两种肿瘤抗原的双重靶向,并显着改善了肿瘤的控制和生存率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号