当前位置:
X-MOL 学术
›
BBA Mol. Basis Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the molecular pathogenesis of cardiospondylocarpofacial syndrome: MAP3K7 c.737-7A > G variant alters the TGFβ-mediated α-SMA cytoskeleton assembly and autophagy.
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.bbadis.2020.165742 Lucia Micale 1 , Silvia Morlino 2 , Tommaso Biagini 3 , Annalucia Carbone 4 , Carmela Fusco 1 , Marco Ritelli 5 , Vincenzo Giambra 6 , Nicoletta Zoppi 5 , Grazia Nardella 7 , Angelantonio Notarangelo 1 , Annalisa Schirizzi 8 , Gianluigi Mazzoccoli 4 , Paola Grammatico 2 , Emma M Wade 9 , Tommaso Mazza 3 , Marina Colombi 5 , Marco Castori 1
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.bbadis.2020.165742 Lucia Micale 1 , Silvia Morlino 2 , Tommaso Biagini 3 , Annalucia Carbone 4 , Carmela Fusco 1 , Marco Ritelli 5 , Vincenzo Giambra 6 , Nicoletta Zoppi 5 , Grazia Nardella 7 , Angelantonio Notarangelo 1 , Annalisa Schirizzi 8 , Gianluigi Mazzoccoli 4 , Paola Grammatico 2 , Emma M Wade 9 , Tommaso Mazza 3 , Marina Colombi 5 , Marco Castori 1
Affiliation
|
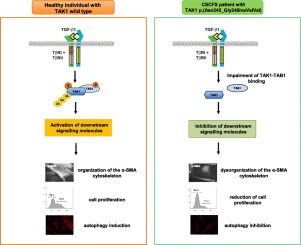
Transforming growth factor beta-activated kinase 1 (TAK1) is a highly conserved kinase protein encoded by MAP3K7, and activated by multiple extracellular stimuli, growth factors and cytokines. Heterozygous variants in MAP3K7 cause the cardiospondylocarpofacial syndrome (CSCFS) which is characterized by short stature, dysmorphic facial features, cardiac septal defects with valve dysplasia, and skeletal anomalies. CSCFS has been described in seven patients to date and its molecular pathogenesis is only partially understood. Here, the functional effects of the MAP3K7 c.737-7A > G variant, previously identified in a girl with CSCFS and additional soft connective tissue features, were explored. This splice variant generates an in-frame insertion of 2 amino acid residues in the kinase domain of TAK1. Computational analysis revealed that this in-frame insertion alters protein dynamics in the kinase activation loop responsible for TAK1 autophosphorylation after binding with its interactor TAB1. Co-immunoprecipitation studies demonstrate that the ectopic expression of TAK1-mutated protein impairs its ability to physically bind TAB1. In patient's fibroblasts, MAP3K7 c.737-7A > G variant results in reduced TAK1 autophosphorylation and dysregulation of the downstream TAK1-dependent signaling pathway. TAK1 loss-of-function is associated with an impaired TGFβ-mediated α-SMA cytoskeleton assembly and cell migration, and defective autophagy process. These findings contribute to our understanding of the molecular pathogenesis of CSCFS and might offer the rationale for the design of novel therapeutic targets.
中文翻译:

对心桥韧带面部综合征的分子发病机理的见解:MAP3K7 c.737-7A> G变体改变了TGFβ介导的α-SMA细胞骨架组装和自噬。
转化生长因子β激活激酶1(TAK1)是由MAP3K7编码的高度保守的激酶蛋白,并被多种细胞外刺激,生长因子和细胞因子激活。MAP3K7中的杂合变异体会导致心桥腕面部综合征(CSCFS),其特征是身材矮小,面部畸形,具有瓣膜发育不良的心脏间隔缺损和骨骼异常。迄今为止,已有7位患者描述了CSCFS,其分子发病机理仅得到部分了解。在这里,探讨了MAP3K7 c.737-7A> G变体的功能作用,该变体先前在具有CSCFS和其他软性结缔组织特征的女孩中鉴定。该剪接变体在TAK1的激酶结构域中在框架内插入2个氨基酸残基。计算分析表明,该框内插入与它的相互作用体TAB1结合后,可改变负责TAK1自磷酸化的激酶激活环中的蛋白质动力学。免疫共沉淀研究表明,TAK1突变蛋白的异位表达削弱了其与TAB1物理结合的能力。在患者的成纤维细胞中,MAP3K7 c.737-7A> G变体导致TAK1自磷酸化水平降低和下游TAK1依赖性信号转导通路失调。TAK1功能丧失与TGFβ介导的α-SMA细胞骨架装配和细胞迁移受损以及自噬过程缺陷有关。这些发现有助于我们对CSCFS分子发病机制的理解,并可能为设计新型治疗靶标提供依据。
更新日期:2020-03-19
中文翻译:

对心桥韧带面部综合征的分子发病机理的见解:MAP3K7 c.737-7A> G变体改变了TGFβ介导的α-SMA细胞骨架组装和自噬。
转化生长因子β激活激酶1(TAK1)是由MAP3K7编码的高度保守的激酶蛋白,并被多种细胞外刺激,生长因子和细胞因子激活。MAP3K7中的杂合变异体会导致心桥腕面部综合征(CSCFS),其特征是身材矮小,面部畸形,具有瓣膜发育不良的心脏间隔缺损和骨骼异常。迄今为止,已有7位患者描述了CSCFS,其分子发病机理仅得到部分了解。在这里,探讨了MAP3K7 c.737-7A> G变体的功能作用,该变体先前在具有CSCFS和其他软性结缔组织特征的女孩中鉴定。该剪接变体在TAK1的激酶结构域中在框架内插入2个氨基酸残基。计算分析表明,该框内插入与它的相互作用体TAB1结合后,可改变负责TAK1自磷酸化的激酶激活环中的蛋白质动力学。免疫共沉淀研究表明,TAK1突变蛋白的异位表达削弱了其与TAB1物理结合的能力。在患者的成纤维细胞中,MAP3K7 c.737-7A> G变体导致TAK1自磷酸化水平降低和下游TAK1依赖性信号转导通路失调。TAK1功能丧失与TGFβ介导的α-SMA细胞骨架装配和细胞迁移受损以及自噬过程缺陷有关。这些发现有助于我们对CSCFS分子发病机制的理解,并可能为设计新型治疗靶标提供依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号