当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibrium Study for the Ternary System of Water + Acetic Acid + 2-Octanol
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jced.9b00954 Lingbo Feng 1, 2 , Wei Zhang 1, 2 , Minglan Ge 1, 2 , Yufeng Yi 1, 2 , Jieming Xiong 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jced.9b00954 Lingbo Feng 1, 2 , Wei Zhang 1, 2 , Minglan Ge 1, 2 , Yufeng Yi 1, 2 , Jieming Xiong 1, 2
Affiliation
|
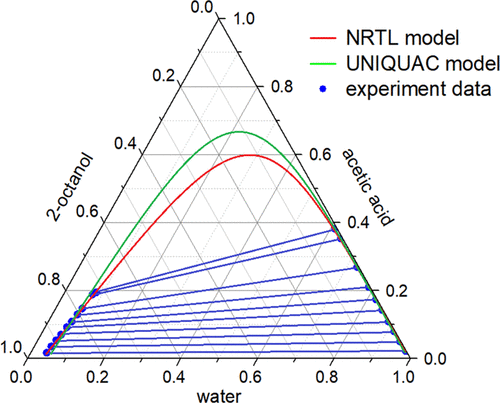
Excellent extraction ability and easy regeneration of the extractant are highly important for the recovery of acetic acid from water by solvent extraction. In this paper, the extraction capability of octanol isomers and some conventional extractants was investigated. It was found that the extraction capability of 2-octanol was significantly better than that of the traditional extractant isopropyl acetate. The liquid–liquid equilibrium data for the ternary system of water + acetic acid + 2-octanol were measured at different temperatures (293.2, 303.2, and 313.2 K) under 101 kPa. The results showed that the distribution coefficient and selectivity decreased with an increase in acetic acid concentration and the temperature had little impact on the distribution coefficient, suggesting that the extraction could be conducted over a large temperature range. The reliability of the equilibrium data was also verified using the Othmer–Tobias and Hand equations, and the correlation coefficients were all close to 1.0, suggesting that the experimental data was reliable. Furthermore, the NRTL (nonrandom two-liquid) and UNIQUAC (universal quasichemical) models were employed to correlate the experimental data, and the binary interaction parameters of the ternary system were obtained. The results indicated that, for the UNIQUAC model, the root-mean-square deviations between the experimental and simulated data at different temperatures were from 0.63% to 0.94% and those for the NRTL model were all within 0.6%, which indicate that the NRTL model is more suitable for the ternary system of water + acetic acid + 2-octanol.
中文翻译:

水+乙酸+ 2-辛醇三元体系的液-液平衡研究
优异的萃取能力和萃取剂的易于再生对于通过溶剂萃取从水中回收乙酸至关重要。本文研究了辛醇异构体和一些常规萃取剂的萃取能力。发现2-辛醇的萃取能力明显优于传统萃取剂乙酸异丙酯。在101 kPa的不同温度(293.2、303.2和313.2 K)下,测量了水+乙酸+ 2-辛醇三元体系的液-液平衡数据。结果表明,随着乙酸浓度的增加,分配系数和选择性降低,温度对分配系数的影响很小;这表明提取可以在较大的温度范围内进行。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。相关系数均接近1.0,说明实验数据可靠。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。相关系数均接近1.0,说明实验数据可靠。此外,采用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。利用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。利用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。
更新日期:2020-02-24
中文翻译:

水+乙酸+ 2-辛醇三元体系的液-液平衡研究
优异的萃取能力和萃取剂的易于再生对于通过溶剂萃取从水中回收乙酸至关重要。本文研究了辛醇异构体和一些常规萃取剂的萃取能力。发现2-辛醇的萃取能力明显优于传统萃取剂乙酸异丙酯。在101 kPa的不同温度(293.2、303.2和313.2 K)下,测量了水+乙酸+ 2-辛醇三元体系的液-液平衡数据。结果表明,随着乙酸浓度的增加,分配系数和选择性降低,温度对分配系数的影响很小;这表明提取可以在较大的温度范围内进行。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。还使用Othmer-Tobias和Hand方程验证了平衡数据的可靠性,并且相关系数都接近1.0,表明实验数据是可靠的。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。相关系数均接近1.0,说明实验数据可靠。此外,采用NRTL(非随机两液)和UNIQUAC(通用准化学)模型对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。相关系数均接近1.0,说明实验数据可靠。此外,采用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。利用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。利用NRTL(非随机两液)模型和UNIQUAC(通用准化学模型)对实验数据进行关联,得到三元体系的二元相互作用参数。结果表明,对于UNIQUAC模型,在不同温度下,实验数据和模拟数据之间的均方根偏差为0.63%至0.94%,而NRTL模型的均方根偏差均在0.6%以内,这表明NRTL该模型更适合于水+乙酸+ 2-辛醇的三元体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号