当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A MORC-driven transcriptional switch controls Toxoplasma developmental trajectories and sexual commitment.
Nature Microbiology ( IF 20.5 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41564-020-0674-4 Dayana C Farhat 1 , Christopher Swale 1 , Céline Dard 1 , Dominique Cannella 1 , Philippe Ortet 2 , Mohamed Barakat 2 , Fabien Sindikubwabo 1 , Lucid Belmudes 3 , Pieter-Jan De Bock 3 , Yohann Couté 3 , Alexandre Bougdour 1 , Mohamed-Ali Hakimi 1
Nature Microbiology ( IF 20.5 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41564-020-0674-4 Dayana C Farhat 1 , Christopher Swale 1 , Céline Dard 1 , Dominique Cannella 1 , Philippe Ortet 2 , Mohamed Barakat 2 , Fabien Sindikubwabo 1 , Lucid Belmudes 3 , Pieter-Jan De Bock 3 , Yohann Couté 3 , Alexandre Bougdour 1 , Mohamed-Ali Hakimi 1
Affiliation
|
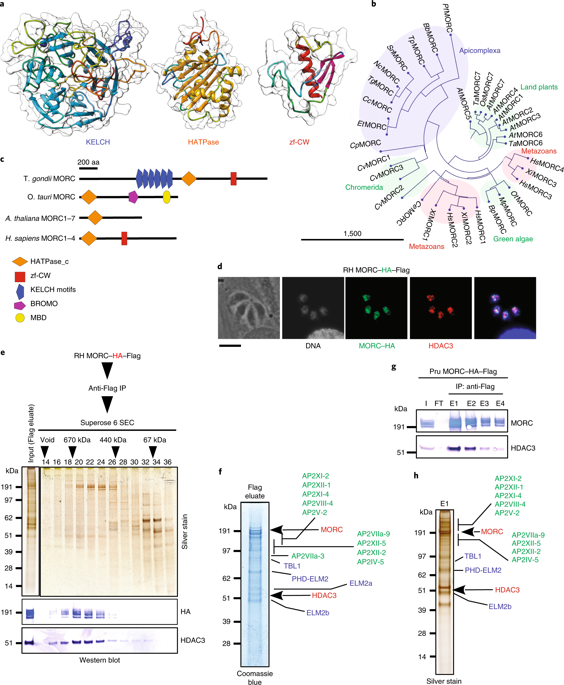
Toxoplasma gondii has a complex life cycle that is typified by asexual development that takes place in vertebrates, and sexual reproduction, which occurs exclusively in felids and is therefore less studied. The developmental transitions rely on changes in the patterns of gene expression, and recent studies have assigned roles for chromatin shapers, including histone modifications, in establishing specific epigenetic programs for each given stage. Here, we identified the T. gondii microrchidia (MORC) protein as an upstream transcriptional repressor of sexual commitment. MORC, in a complex with Apetala 2 (AP2) transcription factors, was shown to recruit the histone deacetylase HDAC3, thereby impeding the accessibility of chromatin at the genes that are exclusively expressed during sexual stages. We found that MORC-depleted cells underwent marked transcriptional changes, resulting in the expression of a specific repertoire of genes, and revealing a shift from asexual proliferation to sexual differentiation. MORC acts as a master regulator that directs the hierarchical expression of secondary AP2 transcription factors, and these transcription factors potentially contribute to the unidirectionality of the life cycle. Thus, MORC plays a cardinal role in the T. gondii life cycle, and its conditional depletion offers a method to study the sexual development of the parasite in vitro, and is proposed as an alternative to the requirement of T. gondii infections in cats.
中文翻译:

MORC驱动的转录开关控制弓形虫的发育轨迹和性承诺。
弓形虫具有复杂的生命周期,其典型特征是发生在脊椎动物中的无性生殖和有性生殖(仅在猫科动物中发生,因此研究较少)。发育过渡依赖于基因表达模式的变化,最近的研究已经为染色质整形剂(包括组蛋白修饰)分配了作用,以建立每个给定阶段的特定表观遗传程序。在这里,我们确定了弓形虫微棘球虫(MORC)蛋白为性承诺的上游转录阻遏物。MORC与Apetala 2(AP2)转录因子形成复合物,显示出募集组蛋白脱乙酰基酶HDAC3,从而阻碍了染色质仅在性阶段表达的基因的可及性。我们发现,耗尽MORC的细胞经历了明显的转录变化,导致特定基因组的表达,并揭示了从无性繁殖向性别分化的转变。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。
更新日期:2020-02-24
中文翻译:

MORC驱动的转录开关控制弓形虫的发育轨迹和性承诺。
弓形虫具有复杂的生命周期,其典型特征是发生在脊椎动物中的无性生殖和有性生殖(仅在猫科动物中发生,因此研究较少)。发育过渡依赖于基因表达模式的变化,最近的研究已经为染色质整形剂(包括组蛋白修饰)分配了作用,以建立每个给定阶段的特定表观遗传程序。在这里,我们确定了弓形虫微棘球虫(MORC)蛋白为性承诺的上游转录阻遏物。MORC与Apetala 2(AP2)转录因子形成复合物,显示出募集组蛋白脱乙酰基酶HDAC3,从而阻碍了染色质仅在性阶段表达的基因的可及性。我们发现,耗尽MORC的细胞经历了明显的转录变化,导致特定基因组的表达,并揭示了从无性繁殖向性别分化的转变。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。MORC充当主要调控因子,指导次级AP2转录因子的分层表达,而这些转录因子潜在地有助于生命周期的单向性。因此,MORC在弓形虫的生命周期中起着主要作用,其条件耗竭为体外研究寄生虫的性发育提供了一种方法,并被提议作为猫对弓形虫感染的一种替代选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号