当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease.
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaneurol.2019.5154 Rik Ossenkoppele 1, 2 , Chul Hyoung Lyoo 3 , Jonas Jester-Broms 1 , Carole H Sudre 4, 5, 6 , Hanna Cho 5 , Young Hoon Ryu 7 , Jae Yong Choi 7, 8 , Ruben Smith 1 , Olof Strandberg 1 , Sebastian Palmqvist 1 , Joel Kramer 9 , Adam L Boxer 9 , Maria L Gorno-Tempini 9 , Bruce L Miller 9 , Renaud La Joie 9 , Gil D Rabinovici 9, 10, 11, 12 , Oskar Hansson 1, 13
JAMA Neurology ( IF 20.4 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaneurol.2019.5154 Rik Ossenkoppele 1, 2 , Chul Hyoung Lyoo 3 , Jonas Jester-Broms 1 , Carole H Sudre 4, 5, 6 , Hanna Cho 5 , Young Hoon Ryu 7 , Jae Yong Choi 7, 8 , Ruben Smith 1 , Olof Strandberg 1 , Sebastian Palmqvist 1 , Joel Kramer 9 , Adam L Boxer 9 , Maria L Gorno-Tempini 9 , Bruce L Miller 9 , Renaud La Joie 9 , Gil D Rabinovici 9, 10, 11, 12 , Oskar Hansson 1, 13
Affiliation
|
Importance
Better understanding is needed of the degree to which individuals tolerate Alzheimer disease (AD)-like pathological tau with respect to brain structure (brain resilience) and cognition (cognitive resilience).
Objective
To examine the demographic (age, sex, and educational level), genetic (APOE-ε4 status), and neuroimaging (white matter hyperintensities and cortical thickness) factors associated with interindividual differences in brain and cognitive resilience to tau positron emission tomography (PET) load and to changes in global cognition over time.
Design, Setting, an Participants
In this cross-sectional, longitudinal study, tau PET was performed from June 1, 2014, to November 30, 2017, and global cognition monitored for a mean [SD] interval of 2.0 [1.8] years at 3 dementia centers in South Korea, Sweden, and the United States. The study included amyloid-β-positive participants with mild cognitive impairment or AD dementia. Data analysis was performed from October 26, 2018, to December 11, 2019.
Exposures
Standard dementia screening, cognitive testing, brain magnetic resonance imaging, amyloid-β PET and cerebrospinal fluid analysis, and flortaucipir (tau) labeled with fluor-18 (18F) PET.
Main Outcomes and Measures
Separate linear regression models were performed between whole cortex [18F]flortaucipir uptake and cortical thickness, and standardized residuals were used to obtain a measure of brain resilience. The same procedure was performed for whole cortex [18F]flortaucipir uptake vs Mini-Mental State Examination (MMSE) as a measure of cognitive resilience. Bivariate and multivariable linear regression models were conducted with age, sex, educational level, APOE-ε4 status, white matter hyperintensity volumes, and cortical thickness as independent variables and brain and cognitive resilience measures as dependent variables. Linear mixed models were performed to examine whether changes in MMSE scores over time differed as a function of a combined brain and cognitive resilience variable.
Results
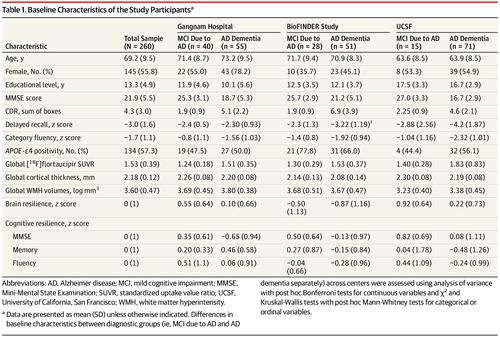
A total of 260 participants (145 [55.8%] female; mean [SD] age, 69.2 [9.5] years; mean [SD] MMSE score, 21.9 [5.5]) were included in the study. In multivariable models, women (standardized β = -0.15, P = .02) and young patients (standardized β = -0.20, P = .006) had greater brain resilience to pathological tau. Higher educational level (standardized β = 0.23, P < .001) and global cortical thickness (standardized β = 0.23, P < .001) were associated with greater cognitive resilience to pathological tau. Linear mixed models indicated a significant interaction of brain resilience × cognitive resilience × time on MMSE (β [SE] = -0.235 [0.111], P = .03), with steepest slopes for individuals with both low brain and cognitive resilience.
Conclusions and Relevance
Results of this study suggest that women and young patients with AD have relative preservation of brain structure when exposed to neocortical pathological tau. Interindividual differences in resilience to pathological tau may be important to disease progression because participants with both low brain and cognitive resilience had the most rapid cognitive decline over time.
中文翻译:

评估与阿尔茨海默病患者脑适应性和病理性Tau的认知适应性相关的人口统计学,遗传和成像变量。
重要性关于脑结构(大脑弹性)和认知(认知弹性),人们需要更好地理解个体对阿尔茨海默病(AD)样病理性tau的耐受程度。目的探讨与个体间脑差异以及对tau正电子发射断层扫描(PET)的认知适应性相关的人口统计学(年龄,性别和教育水平),遗传因素(APOE-ε4状况)和神经影像(白质高信号和皮层厚度)因素),并随着时间的推移改变整体认知度。设计,背景和参与者在这项横断面,纵向研究中,tau PET于2014年6月1日至2017年11月30日进行,全球认知水平在3的平均[SD]间隔为2.0 [1.8]年。痴呆症的研究中心位于韩国,瑞典和美国。该研究包括患有轻度认知障碍或AD痴呆的淀粉样β阳性参与者。从2018年10月26日至2019年12月11日进行数据分析。暴露标准痴呆症筛查,认知测试,脑磁共振成像,β-淀粉样蛋白和脑脊液分析以及以flu-18(18F)标记的氟罗西吡(tau) ) 宠物。主要结果和测量指标在整个皮质[18F]花胶的摄入量与皮质厚度之间进行了单独的线性回归模型,并使用标准化残差获得了脑适应能力的测量指标。整个皮质[18F]预防性花胶的摄取量与迷你精神状态检查(MMSE)进行了相同的操作,以衡量认知弹性。根据年龄,性别,受教育程度,APOE-ε4状态,白质高血容量和皮层厚度为自变量,而脑和认知适应性指标为因变量。进行线性混合模型以检查MMSE分数随时间的变化是否因大脑和认知适应力变量的组合而异。结果研究共纳入260名参与者(女性145 [55.8%];平均[SD]年龄:69.2 [9.5]岁;平均[SD] MMSE分数:21.9 [5.5])。在多变量模型中,女性(标准β= -0.15,P = .02)和年轻患者(标准β= -0.20,P = .006)具有更大的脑适应病理性tau蛋白的能力。较高的教育水平(标准β= 0.23,P <.001)和整体皮质厚度(标准β= 0.23,P <.001)与更大的病理性tau蛋白认知能力相关。线性混合模型表明,脑适应能力×认知适应能力×时间对MMSE有显着的交互作用(β[SE] = -0.235 [0.111],P = .03),对于低脑和认知适应能力的个体,斜率最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮质病理性tau时具有相对保存的脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。对于大脑和认知适应能力低的人来说,坡度最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮层病理性tau时具有相对保存的大脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。对于大脑和认知适应能力低的人来说,坡度最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮层病理性tau时具有相对保存的大脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。
更新日期:2020-05-01
中文翻译:

评估与阿尔茨海默病患者脑适应性和病理性Tau的认知适应性相关的人口统计学,遗传和成像变量。
重要性关于脑结构(大脑弹性)和认知(认知弹性),人们需要更好地理解个体对阿尔茨海默病(AD)样病理性tau的耐受程度。目的探讨与个体间脑差异以及对tau正电子发射断层扫描(PET)的认知适应性相关的人口统计学(年龄,性别和教育水平),遗传因素(APOE-ε4状况)和神经影像(白质高信号和皮层厚度)因素),并随着时间的推移改变整体认知度。设计,背景和参与者在这项横断面,纵向研究中,tau PET于2014年6月1日至2017年11月30日进行,全球认知水平在3的平均[SD]间隔为2.0 [1.8]年。痴呆症的研究中心位于韩国,瑞典和美国。该研究包括患有轻度认知障碍或AD痴呆的淀粉样β阳性参与者。从2018年10月26日至2019年12月11日进行数据分析。暴露标准痴呆症筛查,认知测试,脑磁共振成像,β-淀粉样蛋白和脑脊液分析以及以flu-18(18F)标记的氟罗西吡(tau) ) 宠物。主要结果和测量指标在整个皮质[18F]花胶的摄入量与皮质厚度之间进行了单独的线性回归模型,并使用标准化残差获得了脑适应能力的测量指标。整个皮质[18F]预防性花胶的摄取量与迷你精神状态检查(MMSE)进行了相同的操作,以衡量认知弹性。根据年龄,性别,受教育程度,APOE-ε4状态,白质高血容量和皮层厚度为自变量,而脑和认知适应性指标为因变量。进行线性混合模型以检查MMSE分数随时间的变化是否因大脑和认知适应力变量的组合而异。结果研究共纳入260名参与者(女性145 [55.8%];平均[SD]年龄:69.2 [9.5]岁;平均[SD] MMSE分数:21.9 [5.5])。在多变量模型中,女性(标准β= -0.15,P = .02)和年轻患者(标准β= -0.20,P = .006)具有更大的脑适应病理性tau蛋白的能力。较高的教育水平(标准β= 0.23,P <.001)和整体皮质厚度(标准β= 0.23,P <.001)与更大的病理性tau蛋白认知能力相关。线性混合模型表明,脑适应能力×认知适应能力×时间对MMSE有显着的交互作用(β[SE] = -0.235 [0.111],P = .03),对于低脑和认知适应能力的个体,斜率最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮质病理性tau时具有相对保存的脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。对于大脑和认知适应能力低的人来说,坡度最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮层病理性tau时具有相对保存的大脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。对于大脑和认知适应能力低的人来说,坡度最大。结论与相关性本研究结果表明,AD的女性和年轻患者在暴露于新皮层病理性tau时具有相对保存的大脑结构。个体对病理性tau的适应力差异可能对疾病进展很重要,因为大脑和认知适应力低的参与者随着时间的推移认知衰退最快。











































 京公网安备 11010802027423号
京公网安备 11010802027423号