Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-02-24 , DOI: 10.1038/s41418-020-0517-0 Tirta Mario Djajawi 1, 2 , Lei Liu 1, 2, 3 , Jia-Nan Gong 1, 2 , Allan Shuai Huang 1, 2 , Ming-Jie Luo 1, 2, 4 , Zhen Xu 1, 2 , Toru Okamoto 5 , Melissa J Call 1, 2 , David C S Huang 1, 2 , Mark F van Delft 1, 2
|
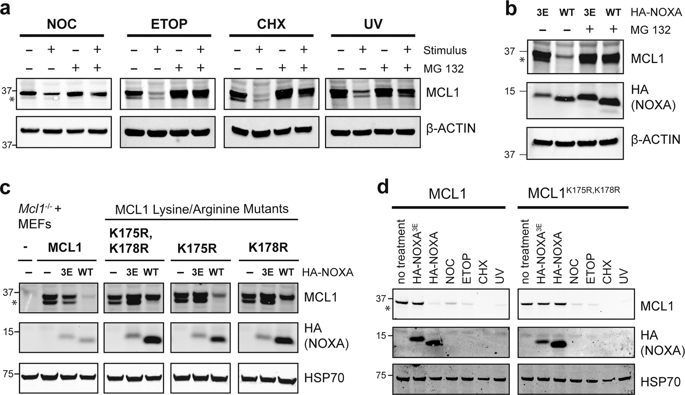
MCL1, a BCL2 relative, is critical for the survival of many cells. Its turnover is often tightly controlled through both ubiquitin-dependent and -independent mechanisms of proteasomal degradation. Several cell stress signals, including DNA damage and cell cycle arrest, are known to elicit distinct E3 ligases to ubiquitinate and degrade MCL1. Another trigger that drives MCL1 degradation is engagement by NOXA, one of its BH3-only protein ligands, but the mechanism responsible has remained unclear. From an unbiased genome-wide CRISPR-Cas9 screen, we discovered that the ubiquitin E3 ligase MARCH5, the ubiquitin E2 conjugating enzyme UBE2K, and the mitochondrial outer membrane protein MTCH2 co-operate to mark MCL1 for degradation by the proteasome—specifically when MCL1 is engaged by NOXA. This mechanism of degradation also required the MCL1 transmembrane domain and distinct MCL1 lysine residues to proceed, suggesting that the components likely act on the MCL1:NOXA complex by associating with it in a specific orientation within the mitochondrial outer membrane. MTCH2 has not previously been reported to regulate protein stability, but is known to influence the mitochondrial localization of certain key apoptosis regulators and to impact metabolism. We have now pinpointed an essential but previously unappreciated role for MTCH2 in turnover of the MCL1:NOXA complex by MARCH5, further strengthening its links to BCL2-regulated apoptosis.
中文翻译:

MARCH5 需要 MTCH2 来协调 MCL1:NOXA 复合物的蛋白酶体转换。
MCL1 是 BCL2 的亲戚,对许多细胞的存活至关重要。它的周转通常通过蛋白酶体降解的泛素依赖性和非依赖性机制受到严格控制。已知几种细胞应激信号,包括 DNA 损伤和细胞周期停滞,会引发不同的 E3 连接酶泛素化和降解 MCL1。另一个驱动 MCL1 降解的触发因素是 NOXA 的参与,NOXA 是其唯一的 BH3 蛋白配体之一,但其机制仍不清楚。从无偏的全基因组 CRISPR-Cas9 筛选中,我们发现泛素 E3 连接酶 MARCH5、泛素 E2 结合酶 UBE2K 和线粒体外膜蛋白 MTCH2 协同作用以标记 MCL1 被蛋白酶体降解 - 特别是当 MCL1 被蛋白酶体降解时由 NOXA 聘用。这种降解机制还需要 MCL1 跨膜结构域和不同的 MCL1 赖氨酸残基才能进行,这表明这些成分可能通过在线粒体外膜内以特定方向与 MCL1:NOXA 复合物结合而作用于 MCL1:NOXA 复合物。MTCH2 以前没有被报道过调节蛋白质稳定性,但已知它会影响某些关键细胞凋亡调节剂的线粒体定位并影响新陈代谢。我们现在已经确定了 MTCH2 在 MARCH5 的 MCL1:NOXA 复合物转换中的一个重要但以前未被重视的作用,进一步加强了它与 BCL2 调节的细胞凋亡的联系。NOXA 复合物通过在线粒体外膜内以特定方向与其结合。MTCH2 以前没有被报道过调节蛋白质稳定性,但已知它会影响某些关键细胞凋亡调节剂的线粒体定位并影响新陈代谢。我们现在已经确定了 MTCH2 在 MARCH5 的 MCL1:NOXA 复合物转换中的一个重要但以前未被重视的作用,进一步加强了它与 BCL2 调节的细胞凋亡的联系。NOXA 复合物通过在线粒体外膜内以特定方向与其结合。MTCH2 以前没有被报道过调节蛋白质稳定性,但已知它会影响某些关键细胞凋亡调节剂的线粒体定位并影响新陈代谢。我们现在已经确定了 MTCH2 在 MARCH5 的 MCL1:NOXA 复合物转换中的一个重要但以前未被重视的作用,进一步加强了它与 BCL2 调节的细胞凋亡的联系。







































 京公网安备 11010802027423号
京公网安备 11010802027423号