当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic Doping and Anion Reconstructed CoF2 Electrocatalyst for Oxygen Evolution Reaction
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-02-23 , DOI: 10.1002/admi.201901939 Qiuchun Dong 1 , Tingting Su 2 , Wei Ge 1 , Yanfang Ren 3 , Yunlong Liu 3 , Wenjun Wang 3 , Qian Wang 1 , Xiaochen Dong 1, 4
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-02-23 , DOI: 10.1002/admi.201901939 Qiuchun Dong 1 , Tingting Su 2 , Wei Ge 1 , Yanfang Ren 3 , Yunlong Liu 3 , Wenjun Wang 3 , Qian Wang 1 , Xiaochen Dong 1, 4
Affiliation
|
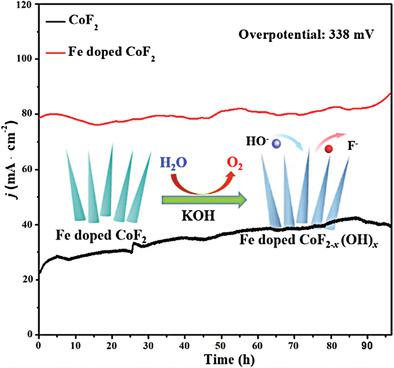
Electrocatalytic water splitting is one of the most promising green solutions for large‐scale hydrogen production, which has developed rapidly in recent years. The slow reaction rate of oxygen evolution reaction (OER) has become the bottleneck to improve the electrocatalytic efficiency. Herein, Fe‐doped CoF2 nanowire arrays on 3D nickel foam are prepared by two steps of hydrothermal reaction and fluorination process. The increased catalytic activity sites and accelerated charge transfer rate make the self‐supported Fe‐doped CoF2 electrode show excellent OER performance in alkaline electrolyte. An overpotential of 230 mV is only required to release a current density of 10 mA cm−2. The Tafel slope derived from the polarization curves is 59 mV dec−1, indicating outstanding electrochemical kinetics performance. A stable output current density of 84 mA cm−2 for more than 96 h is achieved under an invariant overpotential of 338 mV, attributing to the anion reconstruction making part of CoF2 transform into cobalt hydroxide and further improving its electrocatalytic performance.
中文翻译:

原子掺杂和阴离子重构的CoF2电化学催化剂用于氧气释放反应
电催化水分解是大规模制氢最有前景的绿色解决方案之一,近年来发展迅速。放氧反应(OER)的慢反应速率已成为提高电催化效率的瓶颈。在此,通过水热反应和氟化过程两个步骤,制备了在3D镍泡沫上的掺Fe CoF 2纳米线阵列。催化活性位点的增加和电荷转移速率的加快使自支撑的Fe掺杂CoF 2电极在碱性电解质中显示出出色的OER性能。仅需要230 mV的过电势即可释放10 mA cm -2的电流密度。从极化曲线得出的塔菲尔斜率是59 mV dec -1,表明其出色的电化学动力学性能。在恒定的338 mV超电势下,在超过96 h的时间内可获得84 mA cm -2的稳定输出电流密度,这归因于阴离子重建,使部分CoF 2转化为氢氧化钴并进一步提高了其电催化性能。
更新日期:2020-02-23
中文翻译:

原子掺杂和阴离子重构的CoF2电化学催化剂用于氧气释放反应
电催化水分解是大规模制氢最有前景的绿色解决方案之一,近年来发展迅速。放氧反应(OER)的慢反应速率已成为提高电催化效率的瓶颈。在此,通过水热反应和氟化过程两个步骤,制备了在3D镍泡沫上的掺Fe CoF 2纳米线阵列。催化活性位点的增加和电荷转移速率的加快使自支撑的Fe掺杂CoF 2电极在碱性电解质中显示出出色的OER性能。仅需要230 mV的过电势即可释放10 mA cm -2的电流密度。从极化曲线得出的塔菲尔斜率是59 mV dec -1,表明其出色的电化学动力学性能。在恒定的338 mV超电势下,在超过96 h的时间内可获得84 mA cm -2的稳定输出电流密度,这归因于阴离子重建,使部分CoF 2转化为氢氧化钴并进一步提高了其电催化性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号