Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.jmb.2020.02.015 Hebang Yao 1 , Hongmin Cai 1 , Dianfan Li 1
|
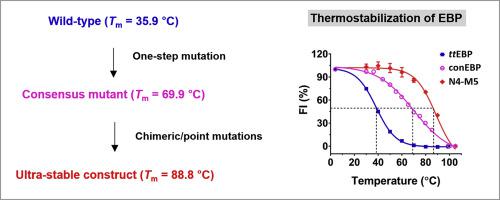
Membrane proteins are generally challenging to work with because of their notorious instability. Protein engineering has been used increasingly to thermostabilize labile membrane proteins such as G-protein–coupled receptors for structural and functional studies in recent years. Two major strategies exist. Scanning mutagenesis systematically eliminates destabilizing residues, whereas the consensus approach assembles mutants with the most frequent residues among selected homologs, bridging sequence conservation with stability. Here, we applied the consensus concept to stabilize a fungal homolog of the human sterol Δ8-7 isomerase, a 26.4 kDa protein with five transmembrane helices. The isomerase is also called emopamil-binding protein (EBP), as it binds this anti-ischemic drug with high affinity. The wild-type had an apparent melting temperature (Tm) of 35.9 °C as measured by the fluorescence-detection size-exclusion chromatography–based thermostability assay. A total of 87 consensus mutations sourced from 22 homologs gained expression level and thermostability, increasing the apparent Tm to 69.9 °C at the cost of partial function loss. Assessing the stability and activity of several systematic chimeric constructs identified a construct with an apparent Tm of 79.8 °C and two regions for function rescue. Further back-mutations of the chimeric construct in the two target regions yielded the final construct with similar apparent activity to the wild-type and an elevated Tm of 88.8 °C, totaling an increase of 52.9 °C. The consensus approach is effective and efficient because it involves fewer constructs compared with scanning mutagenesis. Our results should encourage more use of the consensus strategy for membrane protein thermostabilization.
中文翻译:

通过共识突变膜蛋白的热稳定:真菌Δ8-7甾醇异构酶的案例研究。
膜蛋白通常因其不稳定性而具有挑战性。近年来,越来越多地使用蛋白质工程技术来使不稳定的膜蛋白(如G蛋白偶联受体)热稳定,以进行结构和功能研究。存在两种主要策略。扫描诱变系统地消除了不稳定的残基,而共有方法则在选定的同源物中组装了残基最频繁的突变体,从而稳定地桥接了序列保守性。在这里,我们应用共识性概念来稳定人类固醇Δ8-7异构酶的真菌同源物,该酶是具有五个跨膜螺旋的26.4 kDa蛋白。异构酶也称为Emopamil结合蛋白(EBP),因为它以高亲和力结合了这种抗缺血药物。野生型的熔点很明显(由基于荧光检测尺寸排阻色谱的热稳定性测定法测得的T m)为35.9°C。来自22个同源物的总共87个共有突变获得了表达水平和热稳定性,以部分功能丧失为代价将表观T m增加至69.9°C。评估几种系统嵌合构建体的稳定性和活性后,鉴定出表观T m为79.8°C且有两个区域可拯救功能的构建体。嵌合构建体在两个靶区域中的进一步回突变产生了与野生型具有相似的表观活性和升高的T m的最终构建体最高温度为88.8°C,增加了52.9°C。共识方法是有效和高效的,因为与扫描诱变相比,它涉及的构建体更少。我们的结果应鼓励更多地使用共识性策略进行膜蛋白热稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号