当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fractal self-assembly and aggregation of human amylin
Soft Matter ( IF 2.9 ) Pub Date : 2020/02/24 , DOI: 10.1039/c9sm02463h Suparna Khatun 1, 2, 3, 4, 5 , Anurag Singh 1, 2, 3, 4, 5 , Somnath Maji 3, 4, 5, 6 , Tapas Kumar Maiti 3, 4, 5, 6 , Nisha Pawar 1, 2, 3, 4, 5 , Amar Nath Gupta 1, 2, 3, 4, 5
Soft Matter ( IF 2.9 ) Pub Date : 2020/02/24 , DOI: 10.1039/c9sm02463h Suparna Khatun 1, 2, 3, 4, 5 , Anurag Singh 1, 2, 3, 4, 5 , Somnath Maji 3, 4, 5, 6 , Tapas Kumar Maiti 3, 4, 5, 6 , Nisha Pawar 1, 2, 3, 4, 5 , Amar Nath Gupta 1, 2, 3, 4, 5
Affiliation
|
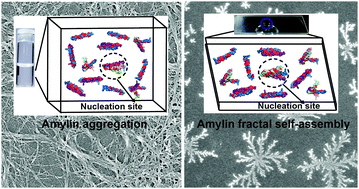
Human amylin is an intrinsically disordered protein believed to have a central role in Type-II diabetes mellitus (T2DM). The formation of intermediate oligomers is a seminal event in the eventual self-assembled fibril structures of amylin. However, the recent experimental investigations have shown the presence of different self-assembled (oligomers, protofilaments, and fibrils) and aggregated structures (amorphous aggregates) of amylin formed during its aggregation. Here, we show that amylin under diffusion-limited conditions leads to fractal self-assembly. The pH and solvent sensitive fractal self-assemblies of amylin were observed using an optical microscope. Confocal microscopy and scanning electron microscopy (SEM) with energy dispersion X-ray analysis (EDAX) were used to confirm the fractal self-assembly of amylin in water and PBS buffer, respectively. The fractal characteristics of the self-assemblies and the aggregates formed during the aggregation of amylin under different pH conditions were investigated using laser light scattering. The hydropathy and the docking study indicated the interactions between the anisotropically distributed hydrophobic residues and polar/ionic residues on the solvent-accessible surface of the protein as the crucial interaction hot-spots for driving the self-assembly and aggregation of human amylin. The simultaneous presence of various self-assemblies of human amylin was observed through different microscopy techniques. The present study may help in designing different fractal-like nanomaterials with potential applications in drug delivery, sensing, and tissue engineering.
中文翻译:

人胰岛淀粉样多肽的分形自组装和聚集
人胰岛淀粉样多肽是一种固有的无序蛋白,被认为在II型糖尿病(T2DM)中起着核心作用。中间寡聚物的形成是胰岛淀粉样多肽最终自我组装的原纤维结构中的一个开创性事件。但是,最近的实验研究表明,淀粉糊精在其聚集过程中形成了不同的自组装体(寡聚物,原丝和原纤维)和聚集结构(无定形聚集体)。在这里,我们表明胰岛淀粉样多肽在扩散受限的条件下导致分形自组装。使用光学显微镜观察胰岛淀粉样多肽的pH和溶剂敏感性分形自组装体。使用共聚焦显微镜和扫描电子显微镜(SEM)以及能量色散X射线分析(EDAX)来确认糊精在水中和PBS缓冲液中的分形自组装,分别。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到了人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。
更新日期:2020-03-26
中文翻译:

人胰岛淀粉样多肽的分形自组装和聚集
人胰岛淀粉样多肽是一种固有的无序蛋白,被认为在II型糖尿病(T2DM)中起着核心作用。中间寡聚物的形成是胰岛淀粉样多肽最终自我组装的原纤维结构中的一个开创性事件。但是,最近的实验研究表明,淀粉糊精在其聚集过程中形成了不同的自组装体(寡聚物,原丝和原纤维)和聚集结构(无定形聚集体)。在这里,我们表明胰岛淀粉样多肽在扩散受限的条件下导致分形自组装。使用光学显微镜观察胰岛淀粉样多肽的pH和溶剂敏感性分形自组装体。使用共聚焦显微镜和扫描电子显微镜(SEM)以及能量色散X射线分析(EDAX)来确认糊精在水中和PBS缓冲液中的分形自组装,分别。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到了人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。利用激光散射研究了不同pH条件下胰岛淀粉样多肽聚合过程中自组装体和聚集体的分形特征。亲水性和对接研究表明,各向异性分布的疏水残基和蛋白质溶剂可及表面上的极性/离子残基之间的相互作用是驱动人胰岛淀粉样多肽自组装和聚集的关键相互作用热点。通过不同的显微镜技术观察到人胰岛淀粉样多肽的各种自组装体的同时存在。本研究可能有助于设计不同的分形纳米材料,在药物输送,传感和组织工程中具有潜在的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号