当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ynamides enabled 6-, 7-, and 8-endo-dig iodocyclization of ethoxyethyl ethers: rapid construction of medium-sized oxacycles at room temperature
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0qo00153h Takashi Okitsu 1, 2, 3 , Akihiro Namura 1, 2, 3 , Shinji Kondo 1, 2, 3 , Shoya Tada 1, 2, 3 , Marina Yanagida 1, 2, 3 , Akimori Wada 1, 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0qo00153h Takashi Okitsu 1, 2, 3 , Akihiro Namura 1, 2, 3 , Shinji Kondo 1, 2, 3 , Shoya Tada 1, 2, 3 , Marina Yanagida 1, 2, 3 , Akimori Wada 1, 2, 3
Affiliation
|
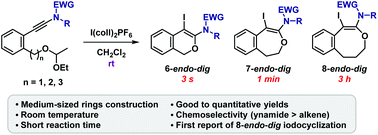
Iodocyclization of ethoxyethyl ethers to ynamides was developed to form six, seven-, and eight-membered cyclic ethers. These reactions reached completion within short reaction times (3 s to 3 h) at room temperature, and the cyclized products were obtained in good to quantitative yields. This is the first report of the construction of medium-sized oxacycles resulting from the iodocyclization of ynamides.
中文翻译:

酰胺可实现乙氧基乙基醚的6、7和8位碘代碘环化:在室温下快速构建中型氧杂环
将乙氧基乙基醚碘化成酰胺,形成六元,七元和八元环状醚。在室温下,这些反应在较短的反应时间内(3 s至3 h)就可以完成,并且获得的环化产物的收率好于定量。这是由乙酰胺的碘环化产生的中等大小草环的首次报道。
更新日期:2020-03-19
中文翻译:

酰胺可实现乙氧基乙基醚的6、7和8位碘代碘环化:在室温下快速构建中型氧杂环
将乙氧基乙基醚碘化成酰胺,形成六元,七元和八元环状醚。在室温下,这些反应在较短的反应时间内(3 s至3 h)就可以完成,并且获得的环化产物的收率好于定量。这是由乙酰胺的碘环化产生的中等大小草环的首次报道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号