当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonium hydroxide as the ultimate amino source for the synthesis of N-unprotected 3-tetrasubstituted aminooxindoles via catalyst-free direct amination
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0gc00130a Jing Yue 1, 2, 3, 4, 5 , Xi-Tao Ma 4, 6, 7 , Xiong-Li Liu 1, 2, 3, 4, 5 , Jun-Xin Wang 3, 4, 5, 8 , Xiong-Wei Liu 1, 2, 3, 4 , Ying Zhou 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0gc00130a Jing Yue 1, 2, 3, 4, 5 , Xi-Tao Ma 4, 6, 7 , Xiong-Li Liu 1, 2, 3, 4, 5 , Jun-Xin Wang 3, 4, 5, 8 , Xiong-Wei Liu 1, 2, 3, 4 , Ying Zhou 1, 2, 3, 4
Affiliation
|
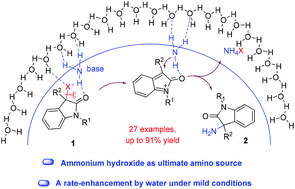
The direct use of ammonium hydroxide in amination for the synthesis of primary amines is considered to be one of the major challenges in synthetic organic chemistry. Herein, the first example of catalyst-free direct amination of 3-halooxindoles with ammonium hydroxide to synthesize N-unprotected 3-tetrasubstituted aminooxindoles in high yields (up to 91% yield) is described. An enhancement of the reaction rate on using water was observed under mild conditions. Such an approach is not only protection/deprotection-free, but also more practical and cost-effective. Moreover, this method could expand the library of 3-tetrasubstituted aminooxindole building blocks for the further synthesis of AG-041R, GHSR antagonists and a charteline unit, opening up a new synthetic pathway in the chemistry of 3-aminooxindoles.
中文翻译:

氢氧化铵是通过无催化剂直接胺化合成N-未保护的3-四取代的氨基恶吲哚的最终氨基来源
在胺化反应中直接使用氢氧化铵合成伯胺被认为是合成有机化学的主要挑战之一。在此,描述了第一个实例,该实例用氢氧化铵无催化地将3-卤代辛多勒胺直接胺化以高产率(高达91%的产率)合成N-未保护的3-四取代的氨基吲哚。在温和的条件下观察到了用水反应速度的提高。这样的方法不仅是无保护/无保护的,而且更加实用和具有成本效益。而且,该方法可以扩展3-四取代的氨基氧吲哚结构单元的文库,用于进一步合成AG-041R,GHSR拮抗剂和沙丁鱼碱单元,为3-氨基氧吲哚的化学开辟了新的合成途径。
更新日期:2020-03-24
中文翻译:

氢氧化铵是通过无催化剂直接胺化合成N-未保护的3-四取代的氨基恶吲哚的最终氨基来源
在胺化反应中直接使用氢氧化铵合成伯胺被认为是合成有机化学的主要挑战之一。在此,描述了第一个实例,该实例用氢氧化铵无催化地将3-卤代辛多勒胺直接胺化以高产率(高达91%的产率)合成N-未保护的3-四取代的氨基吲哚。在温和的条件下观察到了用水反应速度的提高。这样的方法不仅是无保护/无保护的,而且更加实用和具有成本效益。而且,该方法可以扩展3-四取代的氨基氧吲哚结构单元的文库,用于进一步合成AG-041R,GHSR拮抗剂和沙丁鱼碱单元,为3-氨基氧吲哚的化学开辟了新的合成途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号