当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular methionine alkylation constructs sulfonium tethered peptides for protein conjugation.
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0cc00377h Yang Li 1 , Chenshan Lian , Zhanfeng Hou , Dongyuan Wang , Rui Wang , Chuan Wan , Wanjin Zhong , Rongtong Zhao , Yuena Wang , Shuiming Li , Feng Yin , Zigang Li
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0cc00377h Yang Li 1 , Chenshan Lian , Zhanfeng Hou , Dongyuan Wang , Rui Wang , Chuan Wan , Wanjin Zhong , Rongtong Zhao , Yuena Wang , Shuiming Li , Feng Yin , Zigang Li
Affiliation
|
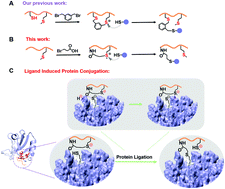
Continuous efforts have been invested in the selective modification of proteins. Herein, we first report the construction of sulfonium tethered cyclic peptides via an intramolecular cyclization by an aliphatic halide. This cyclization could enhance the stability and cellular uptake of peptides. Furthermore, the sulfonium center could be recognized by cysteine in the vicinity of the protein-peptide interacting interface and form a peptide-protein conjugate.
中文翻译:

分子内甲硫氨酸烷基化可构建用于蛋白质缀合的protein束缚肽。
已经对蛋白质的选择性修饰进行了持续的努力。在这里,我们首先报道通过脂族卤化物通过分子内环化来构建sulf系链环肽。这种环化可以增强肽的稳定性和细胞摄取。此外,the中心可以在蛋白-肽相互作用界面附近被半胱氨酸识别并形成肽-蛋白缀合物。
更新日期:2020-03-31
中文翻译:

分子内甲硫氨酸烷基化可构建用于蛋白质缀合的protein束缚肽。
已经对蛋白质的选择性修饰进行了持续的努力。在这里,我们首先报道通过脂族卤化物通过分子内环化来构建sulf系链环肽。这种环化可以增强肽的稳定性和细胞摄取。此外,the中心可以在蛋白-肽相互作用界面附近被半胱氨酸识别并形成肽-蛋白缀合物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号