当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular synthesis of α-aryl β-perfluoroalkyl ketones via N-heterocyclic carbene catalysis.
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0cc00293c Hai-Bin Yang 1 , Zhi-Hou Wang , Jin-Mei Li , Chuande Wu
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/24 , DOI: 10.1039/d0cc00293c Hai-Bin Yang 1 , Zhi-Hou Wang , Jin-Mei Li , Chuande Wu
Affiliation
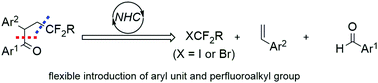
|
A new general de novo synthesis of pharmaceutically important α-aryl β-perfluoroalkyl ketones has been disclosed. Compared with trifluoromethylation-initiated radical 1,2-aryl migration of α,α-diaryl allylic alcohols, this protocol employs a new strategy of biomimetic carbene catalysis to assemble alkene, aldehyde and perfluoroalkyl reagents, providing access to products with excellent flexibility of the aryl unit and perfluoroalkyl group. This method also demonstrates excellent functional group compatibility, including some Grignard reagent sensitive groups.
中文翻译:

通过N-杂环卡宾催化模块化合成α-芳基β-全氟烷基酮。
已经公开了药学上重要的α-芳基β-全氟烷基酮的新的从头合成。与三氟甲基化引发的α,α-二芳基烯丙基醇的自由基1,2-芳基迁移相比,该方案采用了仿生卡宾催化的新策略来组装烯烃,醛和全氟烷基试剂,从而提供了具有优异芳基柔韧性的产品单元和全氟烷基。该方法还证明了极好的官能团相容性,包括一些格氏试剂灵敏基团。
更新日期:2020-03-31
中文翻译:

通过N-杂环卡宾催化模块化合成α-芳基β-全氟烷基酮。
已经公开了药学上重要的α-芳基β-全氟烷基酮的新的从头合成。与三氟甲基化引发的α,α-二芳基烯丙基醇的自由基1,2-芳基迁移相比,该方案采用了仿生卡宾催化的新策略来组装烯烃,醛和全氟烷基试剂,从而提供了具有优异芳基柔韧性的产品单元和全氟烷基。该方法还证明了极好的官能团相容性,包括一些格氏试剂灵敏基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号