Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines.
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-02-24 , DOI: 10.1371/journal.pmed.1003038 Fatima Laher 1 , Zoe Moodie 2 , Kristen W Cohen 2 , Nicole Grunenberg 2 , Linda-Gail Bekker 3 , Mary Allen 4 , Nicole Frahm 2 , Nicole L Yates 5 , Lynn Morris 6, 7 , Mookho Malahleha 8 , Kathryn Mngadi 9 , Brodie Daniels 10 , Craig Innes 11 , Kevin Saunders 5 , Shannon Grant 2 , Chenchen Yu 2 , Peter B Gilbert 2 , Sanjay Phogat 12 , Carlos A DiazGranados 12 , Marguerite Koutsoukos 13 , Olivier Van Der Meeren 13 , Carter Bentley 2 , Nonhlanhla N Mkhize 6, 7 , Michael N Pensiero 4 , Vijay L Mehra 4 , James G Kublin 2 , Lawrence Corey 2 , David C Montefiori 5 , Glenda E Gray 1, 10 , M Juliana McElrath 2 , Georgia D Tomaras 5
PLOS Medicine ( IF 10.5 ) Pub Date : 2020-02-24 , DOI: 10.1371/journal.pmed.1003038 Fatima Laher 1 , Zoe Moodie 2 , Kristen W Cohen 2 , Nicole Grunenberg 2 , Linda-Gail Bekker 3 , Mary Allen 4 , Nicole Frahm 2 , Nicole L Yates 5 , Lynn Morris 6, 7 , Mookho Malahleha 8 , Kathryn Mngadi 9 , Brodie Daniels 10 , Craig Innes 11 , Kevin Saunders 5 , Shannon Grant 2 , Chenchen Yu 2 , Peter B Gilbert 2 , Sanjay Phogat 12 , Carlos A DiazGranados 12 , Marguerite Koutsoukos 13 , Olivier Van Der Meeren 13 , Carter Bentley 2 , Nonhlanhla N Mkhize 6, 7 , Michael N Pensiero 4 , Vijay L Mehra 4 , James G Kublin 2 , Lawrence Corey 2 , David C Montefiori 5 , Glenda E Gray 1, 10 , M Juliana McElrath 2 , Georgia D Tomaras 5
Affiliation
|
BACKGROUND
HVTN 100 evaluated the safety and immunogenicity of an HIV subtype C pox-protein vaccine regimen, investigating a 12-month booster to extend vaccine-induced immune responses.
METHODS AND FINDINGS
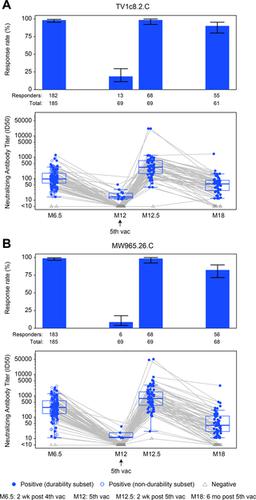
A phase 1-2 randomized double-blind placebo-controlled trial enrolled 252 participants (210 vaccine/42 placebo; median age 23 years; 43% female) between 9 February 2015 and 26 May 2015. Vaccine recipients received ALVAC-HIV (vCP2438) alone at months 0 and 1 and with bivalent subtype C gp120/MF59 at months 3, 6, and 12. Antibody (IgG, IgG3 binding, and neutralizing) and CD4+ T-cell (expressing interferon-gamma, interleukin-2, and CD40 ligand) responses were evaluated at month 6.5 for all participants and at months 12, 12.5, and 18 for a randomly selected subset. The primary analysis compared IgG binding antibody (bAb) responses and CD4+ T-cell responses to 3 vaccine-matched antigens at peak (month 6.5 versus 12.5) and durability (month 12 versus 18) timepoints; IgG responses to CaseA2_gp70_V1V2.B, a primary correlate of risk in RV144, were also compared at these same timepoints. Secondary and exploratory analyses compared IgG3 bAb responses, IgG bAb breadth scores, neutralizing antibody (nAb) responses, antibody-dependent cellular phagocytosis, CD4+ polyfunctionality responses, and CD4+ memory sub-population responses at the same timepoints. Vaccines were generally safe and well tolerated. During the study, there were 2 deaths (both in the vaccine group and both unrelated to study products). Ten participants became HIV-infected during the trial, 7% (3/42) of placebo recipients and 3% (7/210) of vaccine recipients. All 8 serious adverse events were unrelated to study products. Less waning of immune responses was seen after the fifth vaccination than after the fourth, with higher antibody and cellular response rates at month 18 than at month 12: IgG bAb response rates to 1086.C V1V2, 21.0% versus 9.7% (difference = 11.3%, 95% CI = 0.6%-22.0%, P = 0.039), and ZM96.C V1V2, 21.0% versus 6.5% (difference = 14.5%, 95% CI = 4.1%-24.9%, P = 0.004). IgG bAb response rates to all 4 primary V1V2 antigens were higher 2 weeks after the fifth vaccination than 2 weeks after the fourth vaccination: 87.7% versus 75.4% (difference = 12.3%, 95% CI = 1.7%-22.9%, P = 0.022) for 1086.C V1V2, 86.0% versus 63.2% (difference = 22.8%, 95% CI = 9.1%-36.5%, P = 0.001) for TV1c8.2.C V1V2, 67.7% versus 44.6% (difference = 23.1%, 95% CI = 10.4%-35.7%, P < 0.001) for ZM96.C V1V2, and 81.5% versus 60.0% (difference = 21.5%, 95% CI = 7.6%-35.5%, P = 0.002) for CaseA2_gp70_V1V2.B. IgG bAb response rates to the 3 primary vaccine-matched gp120 antigens were all above 90% at both peak timepoints, with no significant differences seen, except a higher response rate to ZM96.C gp120 at month 18 versus month 12: 64.5% versus 1.6% (difference = 62.9%, 95% CI = 49.3%-76.5%, P < 0.001). CD4+ T-cell response rates were higher at month 18 than month 12 for all 3 primary vaccine-matched antigens: 47.3% versus 29.1% (difference = 18.2%, 95% CI = 2.9%-33.4%, P = 0.021) for 1086.C, 61.8% versus 38.2% (difference = 23.6%, 95% CI = 9.5%-37.8%, P = 0.001) for TV1.C, and 63.6% versus 41.8% (difference = 21.8%, 95% CI = 5.1%-38.5%, P = 0.007) for ZM96.C, with no significant differences seen at the peak timepoints. Limitations were that higher doses of gp120 were not evaluated, this study was not designed to investigate HIV prevention efficacy, and the clinical significance of the observed immunological effects is uncertain.
CONCLUSIONS
In this study, a 12-month booster of subtype C pox-protein vaccines restored immune responses, and slowed response decay compared to the 6-month vaccination.
TRIAL REGISTRATION
ClinicalTrials.gov NCT02404311. South African National Clinical Trials Registry (SANCTR number: DOH--27-0215-4796).
中文翻译:

南非 HVTN 100 中未感染 HIV 的健康成人加强 12 个月后的安全性和免疫反应:ALVAC-HIV (vCP2438) 和二价亚型 C gp120/MF59 疫苗的随机双盲安慰剂对照试验。
背景 HVTN 100 评估了 HIV C 亚型痘蛋白疫苗方案的安全性和免疫原性,研究了 12 个月的加强剂以延长疫苗诱导的免疫反应。方法和结果 一项 1-2 期随机双盲安慰剂对照试验在 2015 年 2 月 9 日至 2015 年 5 月 26 日期间招募了 252 名参与者(210 名疫苗/42 名安慰剂;中位年龄 23 岁;43% 为女性)。疫苗接受者接受了 ALVAC-HIV (vCP2438) 在第 0 个月和第 1 个月单独使用,在第 3、6 和 12 个月使用二价亚型 C gp120/MF59。抗体(IgG、IgG3 结合和中和)和 CD4+ T 细胞(表达干扰素-γ、白细胞介素-2)和 CD40 配体)反应在第 6.5 个月对所有参与者进行评估,并在第 12、12.5 和 18 个月对随机选择的子集进行评估。主要分析比较了 IgG 结合抗体 (bAb) 反应和 CD4+ T 细胞对 3 种疫苗匹配抗原在峰值(第 6.5 个月对 12.5 个月)和持久性(第 12 个月对 18 个月)时间点的反应;在这些相同的时间点还比较了对 CaseA2_gp70_V1V2.B 的 IgG 反应,这是 RV144 风险的主要相关因素。二级和探索性分析比较了同一时间点的 IgG3 bAb 反应、IgG bAb 宽度评分、中和抗体 (nAb) 反应、抗体依赖性细胞吞噬作用、CD4+ 多功能反应和 CD4+ 记忆亚群反应。疫苗通常安全且耐受性良好。在研究期间,有 2 人死亡(均在疫苗组中,且均与研究产品无关)。10 名参与者在试验期间感染了 HIV,7% (3/42) 的安慰剂接受者和 3% (7/210) 的疫苗接受者。所有 8 种严重不良事件均与研究产品无关。第 5 次接种后的免疫反应减弱程度低于第 4 次,第 18 个月的抗体和细胞反应率高于第 12 个月:对 1086.C V1V2 的 IgG bAb 反应率,21.0% 与 9.7%(差异 = 11.3 %,95% CI = 0.6%-22.0%,P = 0.039),ZM96.C V1V2,21.0% 与 6.5%(差异 = 14.5%,95% CI = 4.1%-24.9%,P = 0.004)。第 5 次接种后 2 周对所有 4 种主要 V1V2 抗原的 IgG bAb 应答率高于第 4 次接种后 2 周:87.7% 与 75.4%(差异 = 12.3%,95% CI = 1.7%-22.9%,P = 0.022 ) 对于 1086.C V1V2,86.0% 与 63.2%(差异 = 22.8%,95% CI = 9.1%-36.5%,P = 0.001)对于 TV1c8.2.C V1V2,67.7% 与 44。ZM96.C V1V2 为 6%(差异 = 23.1%,95% CI = 10.4%-35.7%,P < 0.001),81.5% 与 60.0%(差异 = 21.5%,95% CI = 7.6%-35.5)对于 CaseA2_gp70_V1V2.B,P = 0.002)。在两个高峰时间点,对 3 种主要疫苗匹配 gp120 抗原的 IgG bAb 应答率均高于 90%,除了第 18 个月与第 12 个月对 ZM96.C gp120 的应答率更高:64.5% 与 1.6 外,没有发现显着差异%(差异 = 62.9%,95% CI = 49.3%-76.5%,P < 0.001)。对于所有 3 种主要疫苗匹配抗原,CD4+ T 细胞应答率在第 18 个月高于第 12 个月:1086 的 47.3% 与 29.1%(差异 = 18.2%,95% CI = 2.9%-33.4%,P = 0.021) .C,TV1.C 为 61.8% 与 38.2%(差异 = 23.6%,95% CI = 9.5%-37.8%,P = 0.001),63.6% 与 41.8%(差异 = 21.8%,95% CI = 5.001) %-38.5%, P = 0.007) 对于 ZM96.C,在高峰时间点没有看到显着差异。局限性在于未评估更高剂量的 gp120,本研究并非旨在调查 HIV 预防功效,并且所观察到的免疫学效应的临床意义尚不确定。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。
更新日期:2020-02-24
中文翻译:

南非 HVTN 100 中未感染 HIV 的健康成人加强 12 个月后的安全性和免疫反应:ALVAC-HIV (vCP2438) 和二价亚型 C gp120/MF59 疫苗的随机双盲安慰剂对照试验。
背景 HVTN 100 评估了 HIV C 亚型痘蛋白疫苗方案的安全性和免疫原性,研究了 12 个月的加强剂以延长疫苗诱导的免疫反应。方法和结果 一项 1-2 期随机双盲安慰剂对照试验在 2015 年 2 月 9 日至 2015 年 5 月 26 日期间招募了 252 名参与者(210 名疫苗/42 名安慰剂;中位年龄 23 岁;43% 为女性)。疫苗接受者接受了 ALVAC-HIV (vCP2438) 在第 0 个月和第 1 个月单独使用,在第 3、6 和 12 个月使用二价亚型 C gp120/MF59。抗体(IgG、IgG3 结合和中和)和 CD4+ T 细胞(表达干扰素-γ、白细胞介素-2)和 CD40 配体)反应在第 6.5 个月对所有参与者进行评估,并在第 12、12.5 和 18 个月对随机选择的子集进行评估。主要分析比较了 IgG 结合抗体 (bAb) 反应和 CD4+ T 细胞对 3 种疫苗匹配抗原在峰值(第 6.5 个月对 12.5 个月)和持久性(第 12 个月对 18 个月)时间点的反应;在这些相同的时间点还比较了对 CaseA2_gp70_V1V2.B 的 IgG 反应,这是 RV144 风险的主要相关因素。二级和探索性分析比较了同一时间点的 IgG3 bAb 反应、IgG bAb 宽度评分、中和抗体 (nAb) 反应、抗体依赖性细胞吞噬作用、CD4+ 多功能反应和 CD4+ 记忆亚群反应。疫苗通常安全且耐受性良好。在研究期间,有 2 人死亡(均在疫苗组中,且均与研究产品无关)。10 名参与者在试验期间感染了 HIV,7% (3/42) 的安慰剂接受者和 3% (7/210) 的疫苗接受者。所有 8 种严重不良事件均与研究产品无关。第 5 次接种后的免疫反应减弱程度低于第 4 次,第 18 个月的抗体和细胞反应率高于第 12 个月:对 1086.C V1V2 的 IgG bAb 反应率,21.0% 与 9.7%(差异 = 11.3 %,95% CI = 0.6%-22.0%,P = 0.039),ZM96.C V1V2,21.0% 与 6.5%(差异 = 14.5%,95% CI = 4.1%-24.9%,P = 0.004)。第 5 次接种后 2 周对所有 4 种主要 V1V2 抗原的 IgG bAb 应答率高于第 4 次接种后 2 周:87.7% 与 75.4%(差异 = 12.3%,95% CI = 1.7%-22.9%,P = 0.022 ) 对于 1086.C V1V2,86.0% 与 63.2%(差异 = 22.8%,95% CI = 9.1%-36.5%,P = 0.001)对于 TV1c8.2.C V1V2,67.7% 与 44。ZM96.C V1V2 为 6%(差异 = 23.1%,95% CI = 10.4%-35.7%,P < 0.001),81.5% 与 60.0%(差异 = 21.5%,95% CI = 7.6%-35.5)对于 CaseA2_gp70_V1V2.B,P = 0.002)。在两个高峰时间点,对 3 种主要疫苗匹配 gp120 抗原的 IgG bAb 应答率均高于 90%,除了第 18 个月与第 12 个月对 ZM96.C gp120 的应答率更高:64.5% 与 1.6 外,没有发现显着差异%(差异 = 62.9%,95% CI = 49.3%-76.5%,P < 0.001)。对于所有 3 种主要疫苗匹配抗原,CD4+ T 细胞应答率在第 18 个月高于第 12 个月:1086 的 47.3% 与 29.1%(差异 = 18.2%,95% CI = 2.9%-33.4%,P = 0.021) .C,TV1.C 为 61.8% 与 38.2%(差异 = 23.6%,95% CI = 9.5%-37.8%,P = 0.001),63.6% 与 41.8%(差异 = 21.8%,95% CI = 5.001) %-38.5%, P = 0.007) 对于 ZM96.C,在高峰时间点没有看到显着差异。局限性在于未评估更高剂量的 gp120,本研究并非旨在调查 HIV 预防功效,并且所观察到的免疫学效应的临床意义尚不确定。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。结论 在本研究中,与 6 个月的疫苗接种相比,12 个月的 C 亚型痘蛋白疫苗加强免疫恢复了免疫反应,并减缓了反应衰减。试验注册 ClinicalTrials.gov NCT02404311。南非国家临床试验登记处(SANCTR 编号:DOH--27-0215-4796)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号