当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed aminocarbonylation of 2-phenyimidazo[1,2-a] pyridines using chloroform as carbon monoxide source and their mechanistic studies
Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-22 , DOI: 10.1016/j.tet.2020.131060 Firdoos Ahmad Sofi , Gurudutt Dubey , Rohit Sharma , Parthasarathi Das , Prasad V. Bharatam
中文翻译:

以氯仿为一氧化碳源的钯催化2-苯并咪唑并[1,2-a]吡啶的氨基羰基化反应及其机理研究
更新日期:2020-02-23
Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-22 , DOI: 10.1016/j.tet.2020.131060 Firdoos Ahmad Sofi , Gurudutt Dubey , Rohit Sharma , Parthasarathi Das , Prasad V. Bharatam
|
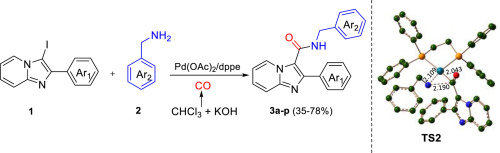
The synthesis of Imidazo[1,2-a]pyridine-3-carboxamides has been carried out by aminocarbonylation using chloroform as carbon monoxide surrogate. Reported protocol is simple, efficient, tolerates wide variety of substrates and the products were formed in good yields. The method can be exploited for the functionalisation of wide range of N-heterocycles with medicinal interest. The detailed mechanistic investigation of metal-catalyzed carboxamide preparation using Density Functional Theory (DFT) calculations has also been reported.
中文翻译:

以氯仿为一氧化碳源的钯催化2-苯并咪唑并[1,2-a]吡啶的氨基羰基化反应及其机理研究
咪唑并[1,2 - a ]吡啶-3-甲酰胺的合成已通过氨基羰基化,使用氯仿作为一氧化碳替代物来进行。所报道的方案简单,有效,耐受各种各样的底物,并且以高收率形成产物。该方法可用于具有医学意义的大范围N-杂环的功能化。还已经报道了使用密度泛函理论(DFT)计算的金属催化羧酰胺制备的详细机理研究。







































 京公网安备 11010802027423号
京公网安备 11010802027423号