Gastroenterology ( IF 29.4 ) Pub Date : 2020-02-22 , DOI: 10.1053/j.gastro.2020.02.030 William J Sandborn 1 , Subrata Ghosh 2 , Julian Panes 3 , Stefan Schreiber 4 , Geert D'Haens 5 , Satoshi Tanida 6 , Jesse Siffledeen 7 , Jeffrey Enejosa 8 , Wen Zhou 8 , Ahmed A Othman 8 , Bidan Huang 8 , Peter D R Higgins 9
|
Background & Aims
We evaluated the efficacy and safety of upadacitinib, an oral selective inhibitor of Janus kinase 1, as induction therapy for ulcerative colitis (UC).
Methods
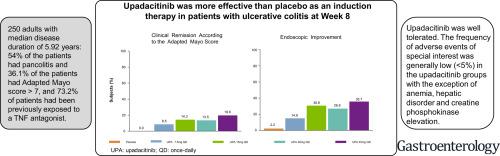
We performed a multicenter, double-blind, phase 2b study of 250 adults with moderately to severely active UC and an inadequate response, loss of response, or intolerance to corticosteroids, immunosuppressive agents, and/or biologic therapies. Patients were randomly assigned to groups that received placebo or induction therapy with upadacitinib (7.5 mg, 15 mg, 30 mg, or 45 mg, extended release), once daily for 8 weeks. The primary endpoint was the proportion of participants who achieve clinical remission according to the adapted Mayo score at week 8. No multiplicity adjustments were applied.
Results
At week 8, 8.5%, 14.3%, 13.5%, and 19.6% of patients receiving 7.5 mg, 15 mg, 30 mg, or 45 mg upadacitinib, respectively, achieved clinical remission compared with none of the patients receiving placebo (P = .052, P = .013, P = .011, and P = .002 compared with placebo, respectively). Endoscopic improvement at week 8, defined as endoscopic subscore of ≤ 1, was achieved in 14.9%, 30.6%, 26.9%, and 35.7% of patients receiving upadacitinib 7.5 mg, 15 mg, 30 mg, or 45 mg, respectively, compared with 2.2% receiving placebo (P = .033, P < .001, P < .001, and P < .001 compared with placebo, respectively). One event of herpes zoster and 1 participant with pulmonary embolism and deep venous thrombosis (diagnosed 26 days after treatment discontinuation) were reported in the group that received upadacitinib 45 mg once daily. Increases in serum lipid levels and creatine phosphokinase with upadacitinib were observed.
Conclusion
In a phase 2b trial, 8 weeks of treatment with upadacitinib was more effective than placebo for inducing remission in patients with moderately to severely active UC. (ClinicalTrials.gov, Number: NCT02819635)
中文翻译:

Upadacitinib 在活动性溃疡性结肠炎患者随机试验中的疗效。
背景与目标
我们评估了 upadacitinib(一种 Janus 激酶 1 的口服选择性抑制剂)作为溃疡性结肠炎 (UC) 诱导疗法的有效性和安全性。
方法
我们对 250 名患有中度至重度活动性 UC 且对皮质类固醇、免疫抑制剂和/或生物疗法反应不足、反应丧失或不耐受的成人进行了一项多中心、双盲、2b 期研究。患者被随机分配到接受安慰剂或 upadacitinib(7.5 mg、15 mg、30 mg 或 45 mg,缓释)诱导治疗的组,每天一次,持续 8 周。主要终点是根据第 8 周调整后的 Mayo 评分达到临床缓解的参与者比例。未应用多重性调整。
结果
在第 8 周,接受 7.5 mg、15 mg、30 mg 或 45 mg upadacitinib 的患者中分别有 8.5%、14.3%、13.5% 和 19.6% 的患者达到了临床缓解,而接受安慰剂的患者均未达到(P = . 052、P = .013、P = .011 和P = .002,分别与安慰剂相比)。与接受 upadacitinib 7.5 mg、15 mg、30 mg 或 45 mg 治疗的患者相比,第 8 周内镜下改善(定义为内镜评分≤ 1)的患者分别有 14.9%、30.6%、26.9% 和 35.7% 2.2% 接受安慰剂(P = .033、P < .001、P < .001 和P< .001,分别与安慰剂相比)。在接受 upadacitinib 45 mg 每天一次的组中报告了 1 起带状疱疹事件和 1 名患有肺栓塞和深静脉血栓形成的参与者(在停止治疗后 26 天诊断)。观察到用 upadacitinib 增加血清脂质水平和肌酸磷酸激酶。
结论
在 2b 期试验中,upadacitinib 治疗 8 周比安慰剂更有效地诱导中度至重度活动性 UC 患者的缓解。(ClinicalTrials.gov,编号:NCT02819635)



























 京公网安备 11010802027423号
京公网安备 11010802027423号