当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How CuCl and CuCl2 Insert into C-N Bonds of Diazo Compounds: An Electronic Structure and Mechanistic Study.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jpca.9b11991 Fengyu Li 1 , John Zenghui Zhang 1, 2 , Fei Xia 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-04 , DOI: 10.1021/acs.jpca.9b11991 Fengyu Li 1 , John Zenghui Zhang 1, 2 , Fei Xia 1, 2
Affiliation
|
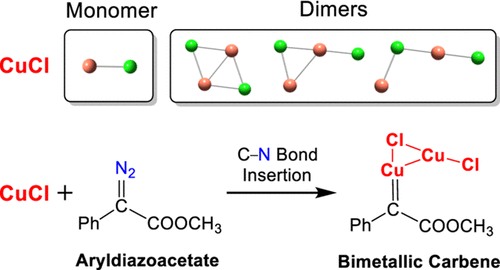
The transition-metal Cu catalysts CuCl and CuCl2 have been widely employed to catalyze a series of chemical reactions with diazo compounds because of their high efficiency and selectivity. However, how to yield the active Cu carbene species from the Cu catalysts and diazo compounds still remains unclear. In this work, we performed a comprehensive theoretical investigation on the electronic structures of CuCl and CuCl2 in solution. The results indicate that the most stable structures for CuCl and CuCl2 are dimer and monomer, respectively. The C-N bond insertion of aryldiazoacetate by CuCl yields a stable bimetallic carbene species, which differs from the monometallic carbene generated from CuCl2.
中文翻译:

CuCl和CuCl2如何插入重氮化合物的CN键中:电子结构和机理研究。
过渡金属Cu催化剂CuCl和CuCl2因其高效和高选择性而被广泛用于催化与重氮化合物的一系列化学反应。然而,如何从Cu催化剂和重氮化合物产生活性Cu卡宾物质仍然不清楚。在这项工作中,我们对溶液中CuCl和CuCl2的电子结构进行了全面的理论研究。结果表明,CuCl和CuCl2最稳定的结构分别是二聚体和单体。CuCl的CN2插入芳基重氮乙酸酯产生稳定的双金属卡宾物种,这与CuCl2生成的单金属卡宾不同。
更新日期:2020-03-04
中文翻译:

CuCl和CuCl2如何插入重氮化合物的CN键中:电子结构和机理研究。
过渡金属Cu催化剂CuCl和CuCl2因其高效和高选择性而被广泛用于催化与重氮化合物的一系列化学反应。然而,如何从Cu催化剂和重氮化合物产生活性Cu卡宾物质仍然不清楚。在这项工作中,我们对溶液中CuCl和CuCl2的电子结构进行了全面的理论研究。结果表明,CuCl和CuCl2最稳定的结构分别是二聚体和单体。CuCl的CN2插入芳基重氮乙酸酯产生稳定的双金属卡宾物种,这与CuCl2生成的单金属卡宾不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号