当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed diastereo- and enantioselective allylic alkylation of oxazolones with 1,3-dienes under base-free conditions.
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/23 , DOI: 10.1039/d0cc00265h Haijian Yang 1 , Dong Xing
Chemical Communications ( IF 4.3 ) Pub Date : 2020/02/23 , DOI: 10.1039/d0cc00265h Haijian Yang 1 , Dong Xing
Affiliation
|
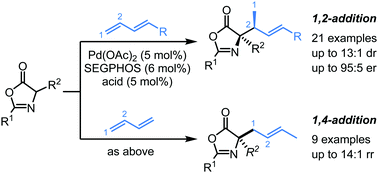
Herein, we report a highly diastereo- and enantioselective allylic alkylation of oxazolones with 1,3-dienes by palladium-hydride catalyst under base-free conditions. With DTBM-SEGPHOS as the chiral ligand, a series of enantioenriched oxazolones bearing tertiary carbon centers were synthesized from substituted 1,3-dienes via exclusive 1,2-addition with moderate to good diastereoselectivities and high enantioselectivities. When simple 1,3-butadiene was used as the allyl precursor under this base-free catalytic system, 1,4-addition products were obtained in good yields with high regioselectivities.
中文翻译:

在无碱条件下,钯催化1,3-二烯与恶唑酮的非对映和对映选择性烯丙基烷基化反应。
在此,我们报道了在无碱条件下,钯氢化物催化的1,3-二烯与恶唑酮的非对映体和对映体选择性烯丙基烷基化。以DTBM-SEGPHOS为手性配体,由取代的1,3-二烯经专门的1,2-加成,由中等至良好的非对映选择性和高对映选择性合成了一系列带有叔碳中心的对映体富集的恶唑酮。当在这种无碱催化体系下,简单的1,3-丁二烯用作烯丙基前体时,可以高产率获得具有高区域选择性的1,4-加成产物。
更新日期:2020-03-31
中文翻译:

在无碱条件下,钯催化1,3-二烯与恶唑酮的非对映和对映选择性烯丙基烷基化反应。
在此,我们报道了在无碱条件下,钯氢化物催化的1,3-二烯与恶唑酮的非对映体和对映体选择性烯丙基烷基化。以DTBM-SEGPHOS为手性配体,由取代的1,3-二烯经专门的1,2-加成,由中等至良好的非对映选择性和高对映选择性合成了一系列带有叔碳中心的对映体富集的恶唑酮。当在这种无碱催化体系下,简单的1,3-丁二烯用作烯丙基前体时,可以高产率获得具有高区域选择性的1,4-加成产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号