当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-23 , DOI: 10.1016/j.freeradbiomed.2020.02.021 Marie Angèle Cucci 1 , Margherita Grattarola 1 , Chiara Dianzani 2 , Giovanna Damia 3 , Francesca Ricci 3 , Antonella Roetto 1 , Francesco Trotta 4 , Giuseppina Barrera 1 , Stefania Pizzimenti 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-23 , DOI: 10.1016/j.freeradbiomed.2020.02.021 Marie Angèle Cucci 1 , Margherita Grattarola 1 , Chiara Dianzani 2 , Giovanna Damia 3 , Francesca Ricci 3 , Antonella Roetto 1 , Francesco Trotta 4 , Giuseppina Barrera 1 , Stefania Pizzimenti 1
Affiliation
|
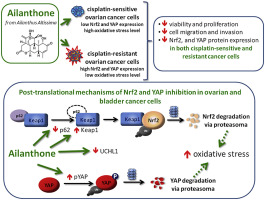
Chemoresistance represents one of the main obstacles in treating several types of cancer, including bladder and ovarian cancers, and it is characterized by an increase of cellular antioxidant potential. Nrf2 and YAP proteins play an important role in increasing chemoresistance and in inducing antioxidant enzymes. It has been reported that Ailanthone (Aila), a compound extracted from the Ailanthus Altissima, has an anticancer activity toward several cancer cell lines, including chemo-resistant cell lines. We have examined the effect of Aila on proliferation, migration and expression of Nrf2 and YAP proteins in A2780 (CDDP-sensitive) and A2780/CP70 (CDDP-resistant) ovarian cancer cells. Furthermore, to clarify the mechanism of Aila action we extended our studies to sensitive and CDDP-resistant 253J-BV bladder cancer cells, which have been used in a previous study on the effect of Aila. Results demonstrated that Aila exerted an inhibitory effect on growth and colony formation of sensitive and CDDP-resistant ovarian cancer cells and reduced oriented cell migration with higher effectiveness in CDDP resistant cells. Moreover, Aila strongly reduced Nrf2 and YAP protein expression and reduced the expression of the Nrf2 target GSTA4, and the YAP/TEAD target survivin. In CDDP-resistant ovarian and bladder cancer cells the intracellular oxidative stress level was lower with respect to the sensitive cells. Moreover, Aila treatment further reduced the superoxide anion content of CDDP-resistant cells in correlation with the reduction of Nrf2 and YAP proteins. However, Aila treatment increased Nrf2 and YAP mRNA expression in all cancer cell lines. The inhibition of proteolysis by MG132, a proteasoma inhibitor, restored Nrf2 and YAP protein expressions, suggesting that the Aila effect was at post-translational level. In accordance with this observation, we found an increase of the Nrf2 inhibitor Keap1, a reduction of p62/SQSTM1, a Nrf2 target which leads Keap1 protein to the autophagic degradation, and a reduction of P-YAP. Moreover, UCHL1 deubiquitinase expression, which was increased in bladder and ovarian resistant cells, was down-regulated by Aila treatment. In conclusion we demonstrated that Aila can reduce proliferation and migration of cancer cells through a mechanism involving a post translational reduction of Nrf2 and YAP proteins which, in turn, entailed an increase of oxidative stress particularly in the chemoresistant lines.
中文翻译:

苯乙酮通过翻译后机制抑制Nrf2和YAP表达,从而增加CDDP抗性卵巢癌细胞和膀胱癌细胞的氧化应激。
化学抗性是治疗几种类型的癌症(包括膀胱癌和卵巢癌)的主要障碍之一,其特点是细胞抗氧化剂的潜力增加。Nrf2和YAP蛋白在增加化学抗性和诱导抗氧化酶方面起着重要作用。据报道,从臭椿中提取的化合物艾兰酮(Aila)对几种癌细胞系,包括化学抗性细胞系具有抗癌活性。我们已经检查了Aila对A2780(CDDP敏感)和A2780 / CP70(CDDP耐药)卵巢癌细胞中Nrf2和YAP蛋白增殖,迁移和表达的影响。此外,为了阐明Aila作用的机制,我们将研究扩展到了敏感且具有CDDP耐药性的253J-BV膀胱癌细胞,在先前关于艾拉效果的研究中已经使用过。结果表明,Aila对敏感的CDDP耐药卵巢癌细胞的生长和集落形成具有抑制作用,并减少了定向细胞迁移,在CDDP耐药细胞中具有更高的效力。此外,Aila大大降低了Nrf2和YAP蛋白的表达,并降低了Nrf2靶标GSTA4和YAP / TEAD靶标survivin的表达。在抗CDDP的卵巢癌细胞和膀胱癌细胞中,相对于敏感细胞,细胞内氧化应激水平较低。此外,与Nrf2和YAP蛋白的减少相关,Aila处理进一步降低了CDDP抗性细胞的超氧阴离子含量。但是,Aila处理增加了所有癌细胞系中Nrf2和YAP mRNA的表达。蛋白酶抑制剂MG132抑制蛋白水解,恢复了Nrf2和YAP蛋白的表达,表明Aila的作用在翻译后水平。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。还原Nrf2和YAP蛋白表达,表明Aila效应是在翻译后的水平。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。还原Nrf2和YAP蛋白表达,表明Aila效应是在翻译后的水平。根据该观察结果,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。Aila处理下调了膀胱和卵巢抗性细胞中的高表达。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。Aila处理下调了膀胱和卵巢抗性细胞中的高表达。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。
更新日期:2020-02-23
中文翻译:

苯乙酮通过翻译后机制抑制Nrf2和YAP表达,从而增加CDDP抗性卵巢癌细胞和膀胱癌细胞的氧化应激。
化学抗性是治疗几种类型的癌症(包括膀胱癌和卵巢癌)的主要障碍之一,其特点是细胞抗氧化剂的潜力增加。Nrf2和YAP蛋白在增加化学抗性和诱导抗氧化酶方面起着重要作用。据报道,从臭椿中提取的化合物艾兰酮(Aila)对几种癌细胞系,包括化学抗性细胞系具有抗癌活性。我们已经检查了Aila对A2780(CDDP敏感)和A2780 / CP70(CDDP耐药)卵巢癌细胞中Nrf2和YAP蛋白增殖,迁移和表达的影响。此外,为了阐明Aila作用的机制,我们将研究扩展到了敏感且具有CDDP耐药性的253J-BV膀胱癌细胞,在先前关于艾拉效果的研究中已经使用过。结果表明,Aila对敏感的CDDP耐药卵巢癌细胞的生长和集落形成具有抑制作用,并减少了定向细胞迁移,在CDDP耐药细胞中具有更高的效力。此外,Aila大大降低了Nrf2和YAP蛋白的表达,并降低了Nrf2靶标GSTA4和YAP / TEAD靶标survivin的表达。在抗CDDP的卵巢癌细胞和膀胱癌细胞中,相对于敏感细胞,细胞内氧化应激水平较低。此外,与Nrf2和YAP蛋白的减少相关,Aila处理进一步降低了CDDP抗性细胞的超氧阴离子含量。但是,Aila处理增加了所有癌细胞系中Nrf2和YAP mRNA的表达。蛋白酶抑制剂MG132抑制蛋白水解,恢复了Nrf2和YAP蛋白的表达,表明Aila的作用在翻译后水平。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。还原Nrf2和YAP蛋白表达,表明Aila效应是在翻译后的水平。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。还原Nrf2和YAP蛋白表达,表明Aila效应是在翻译后的水平。根据该观察结果,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。根据此观察,我们发现Nrf2抑制剂Keap1的增加,p62 / SQSTM1的减少,导致Keap1蛋白自噬降解的Nrf2靶点和P-YAP的减少。而且,在膀胱和卵巢耐药细胞中增加的UCHL1去泛素化酶表达被Aila处理下调。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。Aila处理下调了膀胱和卵巢抗性细胞中的高表达。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。Aila处理下调了膀胱和卵巢抗性细胞中的高表达。总而言之,我们证明了Aila可以通过涉及Nrf2和YAP蛋白翻译后还原的机制来减少癌细胞的增殖和迁移,这反过来又引起了氧化应激的增加,特别是在化学抗性系中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号