当前位置:
X-MOL 学术
›
Appl. Clay. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intrinsic hydrophobicity of smectite basal surfaces quantitatively probed by molecular dynamics simulations
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.clay.2020.105497 Marek Szczerba , Andrey G. Kalinichev , Mariola Kowalik
Applied Clay Science ( IF 5.3 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.clay.2020.105497 Marek Szczerba , Andrey G. Kalinichev , Mariola Kowalik
|
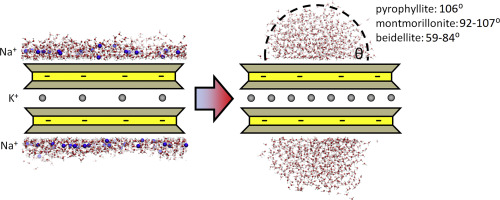
Abstract The siloxane surface of uncharged clays is known to be hydrophobic, which is supported by strong experimental and theoretical evidence. For the siloxane surface of charged clays, like smectites, the picture is not as clear. We are aiming to clarify this issue by molecular simulations in which smectite surface hydrophobicity is quantified through the separate contribution of the surface itself, and the contribution due to the presence of charge-balancing cations on the surface. In order to explore systematically the effects of the total smectite charge and its distribution in the structure, a series of molecular dynamics (MD) simulations was performed for several models of dioctahedral smectites and compared with the results for uncharged pyrophyllite. The largest difference between the simulation results for smectite models with naturally present surface counterions and the models where these ions were artificially removed from the surface, while maintaining the same total charge balance of the model, is in the shape of the water coverage. In the former case, full surface wetting is observed and a relatively flat water film is forming on the surface. Its irregularity and thickness is connected with number of ions on the surface. However, in all cases of smectite surfaces artificially devoid of ions, a water droplet is always formed and the wetting is incomplete. The contact angles of the water droplets on charged montmorillonites are very similar to that on uncharged pyrophyllite surface and range roughly between 110o and 90o. These angles are also affected by the distribution of the octahedral and tetrahedral substitutions in the structure and by their ratio. In the case of purely tetrahedral substitutions the contact angle on the bare smectite surface can be as low as ~60o, but still far from complete wetting. The angular distributions of the H2O dipole vectors as a function of distance from the smectite surface show two preferred surface-oriented types of water molecules when counterions are present, and the total surface is highly hydrophilic. However, for surfaces devoid of ions, a population with dipole angles close to ~90o is dominating, and the smectite surfaces can be considered hydrophobic. It can be thus concluded that, independent of the structural charge, bare smectite surfaces by themselves are either hydrophobic or only moderately hydrophilic. Their experimentally observed highly hydrophilic character is almost entirely due to the charge balancing cations present on the surface.
中文翻译:

通过分子动力学模拟定量探测蒙脱石基底表面的固有疏水性
摘要 已知不带电粘土的硅氧烷表面是疏水的,这得到了强有力的实验和理论证据的支持。对于带电粘土的硅氧烷表面,如蒙脱石,图片并不那么清晰。我们的目标是通过分子模拟来澄清这个问题,其中蒙脱石表面疏水性通过表面本身的单独贡献以及由于表面上电荷平衡阳离子的存在而量化。为了系统地探索总蒙脱石电荷及其在结构中分布的影响,对几种双八面体蒙脱石模型进行了一系列分子动力学 (MD) 模拟,并与不带电荷的叶蜡石的结果进行了比较。具有天然存在的表面反离子的蒙脱石模型与这些离子从表面人工去除的模型之间的最大差异,同时保持模型的相同总电荷平衡,是水覆盖的形状。在前一种情况下,观察到整个表面润湿并且在表面上形成相对平坦的水膜。它的不规则性和厚度与表面离子的数量有关。然而,在所有人工缺乏离子的蒙脱石表面的情况下,总是形成水滴并且润湿不完全。带电蒙脱石上的水滴接触角与不带电的叶蜡石表面的水滴接触角非常相似,范围大致在 110 度到 90 度之间。这些角度还受到结构中八面体和四面体取代基分布及其比率的影响。在纯四面体置换的情况下,裸露的蒙脱石表面的接触角可低至~60°,但仍远未完全润湿。H2O 偶极子矢量的角分布作为距蒙脱石表面距离的函数,当存在抗衡离子时,显示出两种优选的表面取向类型的水分子,并且总表面高度亲水。然而,对于没有离子的表面,偶极角接近 ~90o 的群体占主导地位,蒙脱石表面可以被认为是疏水的。因此可以得出结论,独立于结构电荷,裸露的蒙脱石表面本身要么是疏水的,要么只是适度亲水的。
更新日期:2020-04-01
中文翻译:

通过分子动力学模拟定量探测蒙脱石基底表面的固有疏水性
摘要 已知不带电粘土的硅氧烷表面是疏水的,这得到了强有力的实验和理论证据的支持。对于带电粘土的硅氧烷表面,如蒙脱石,图片并不那么清晰。我们的目标是通过分子模拟来澄清这个问题,其中蒙脱石表面疏水性通过表面本身的单独贡献以及由于表面上电荷平衡阳离子的存在而量化。为了系统地探索总蒙脱石电荷及其在结构中分布的影响,对几种双八面体蒙脱石模型进行了一系列分子动力学 (MD) 模拟,并与不带电荷的叶蜡石的结果进行了比较。具有天然存在的表面反离子的蒙脱石模型与这些离子从表面人工去除的模型之间的最大差异,同时保持模型的相同总电荷平衡,是水覆盖的形状。在前一种情况下,观察到整个表面润湿并且在表面上形成相对平坦的水膜。它的不规则性和厚度与表面离子的数量有关。然而,在所有人工缺乏离子的蒙脱石表面的情况下,总是形成水滴并且润湿不完全。带电蒙脱石上的水滴接触角与不带电的叶蜡石表面的水滴接触角非常相似,范围大致在 110 度到 90 度之间。这些角度还受到结构中八面体和四面体取代基分布及其比率的影响。在纯四面体置换的情况下,裸露的蒙脱石表面的接触角可低至~60°,但仍远未完全润湿。H2O 偶极子矢量的角分布作为距蒙脱石表面距离的函数,当存在抗衡离子时,显示出两种优选的表面取向类型的水分子,并且总表面高度亲水。然而,对于没有离子的表面,偶极角接近 ~90o 的群体占主导地位,蒙脱石表面可以被认为是疏水的。因此可以得出结论,独立于结构电荷,裸露的蒙脱石表面本身要么是疏水的,要么只是适度亲水的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号