当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: Towards a new approach for the management of Parkinson's disease.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-02-21 , DOI: 10.1016/j.jconrel.2020.02.033 Edilson Ribeiro de Oliveira Junior 1 , Eleonora Truzzi 2 , Luca Ferraro 3 , Marco Fogagnolo 4 , Barbara Pavan 5 , Sarah Beggiato 6 , Cecilia Rustichelli 2 , Eleonora Maretti 2 , Eliana Martins Lima 1 , Eliana Leo 2 , Alessandro Dalpiaz 4
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-02-21 , DOI: 10.1016/j.jconrel.2020.02.033 Edilson Ribeiro de Oliveira Junior 1 , Eleonora Truzzi 2 , Luca Ferraro 3 , Marco Fogagnolo 4 , Barbara Pavan 5 , Sarah Beggiato 6 , Cecilia Rustichelli 2 , Eleonora Maretti 2 , Eliana Martins Lima 1 , Eliana Leo 2 , Alessandro Dalpiaz 4
Affiliation
|
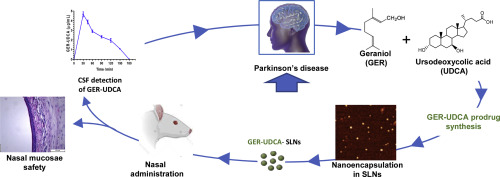
The combined use of different therapeutic agents in the treatment of neurodegenerative disorders is a promising strategy to halt the disease progression. In this context, we aimed to combine the anti-inflammatory properties of geraniol (GER) with the mitochondrial rescue effects of ursodeoxycholic acid (UDCA) in a newly-synthesized prodrug, GER-UDCA, a potential candidate against Parkinson's disease (PD). GER-UDCA was successfully synthetized and characterized in vitro for its ability to release the active compounds in physiological environments. Because of its very poor solubility, GER-UDCA was entrapped into both lipid (SLNs) and polymeric (NPs) nanoparticles in order to explore nose-to-brain pathway towards brain targeting. Both GER-UDCA nanocarriers displayed size below 200 nm, negative zeta potential and the ability to increase the aqueous dissolution rate of the prodrug. As SLNs exhibited the higher GER-UDCA dissolution rate, this formulation was selected for the in vivo GER-UDCA brain targeting experiments. The nasal administration of GER-UDCA-SLNs (1 mg/kg of GER-UDCA) allowed to detect the prodrug in rat cerebrospinal fluid (concentration range = 1.1 to 4.65 μg/mL, 30-150 min after the administration), but not in the bloodstream, thus suggesting the direct nose to brain delivery of the prodrug. Finally, histopathological evaluation demonstrated that, in contrast to the pure GER, nasal administration of GER-UDCA-SLNs did not damage the structural integrity of the nasal mucosa. In conclusion, the present data suggest that GER-UDCA-SLNs could provide an effective and non-invasive approach to boost the access of GER and UDCA to the brain with low dosages.
中文翻译:

纳米囊化的香叶醇/熊去氧胆酸共轭物的鼻腔给药:迈向治疗帕金森氏病的新方法。
在神经退行性疾病的治疗中联合使用不同的治疗剂是阻止疾病进展的有前途的策略。在这种情况下,我们旨在将香叶醇(GER)的抗炎特性与熊去氧胆酸(UDCA)的线粒体拯救作用结合在新合成的前药GER-UDCA中,GER-UDCA是对抗帕金森氏病(PD)的潜在候选药物。GER-UDCA在体外成功合成和表征了其在生理环境中释放活性化合物的能力。由于GER-UDCA的溶解性非常差,因此被包裹在脂质(SLN)和聚合物(NPs)纳米颗粒中,以探索从鼻到脑的大脑靶向途径。两种GER-UDCA纳米载体的尺寸均小于200 nm,负ζ电势和增加前药在水中溶解速率的能力。由于SLNs表现出较高的GER-UDCA溶出速率,因此选择该制剂用于体内GER-UDCA脑靶向实验。鼻腔给药GER-UDCA-SLNs(1 mg / kg GER-UDCA)可以检测大鼠脑脊液中的前药(浓度范围为1.1至4.65μg/ mL,给药后30-150分钟),但不能在血液中,因此提示前药直接通过鼻子输送到大脑。最后,组织病理学评估表明,与纯GER相比,GER-UDCA-SLN的鼻腔给药不会损害鼻黏膜的结构完整性。结论,
更新日期:2020-02-21
中文翻译:

纳米囊化的香叶醇/熊去氧胆酸共轭物的鼻腔给药:迈向治疗帕金森氏病的新方法。
在神经退行性疾病的治疗中联合使用不同的治疗剂是阻止疾病进展的有前途的策略。在这种情况下,我们旨在将香叶醇(GER)的抗炎特性与熊去氧胆酸(UDCA)的线粒体拯救作用结合在新合成的前药GER-UDCA中,GER-UDCA是对抗帕金森氏病(PD)的潜在候选药物。GER-UDCA在体外成功合成和表征了其在生理环境中释放活性化合物的能力。由于GER-UDCA的溶解性非常差,因此被包裹在脂质(SLN)和聚合物(NPs)纳米颗粒中,以探索从鼻到脑的大脑靶向途径。两种GER-UDCA纳米载体的尺寸均小于200 nm,负ζ电势和增加前药在水中溶解速率的能力。由于SLNs表现出较高的GER-UDCA溶出速率,因此选择该制剂用于体内GER-UDCA脑靶向实验。鼻腔给药GER-UDCA-SLNs(1 mg / kg GER-UDCA)可以检测大鼠脑脊液中的前药(浓度范围为1.1至4.65μg/ mL,给药后30-150分钟),但不能在血液中,因此提示前药直接通过鼻子输送到大脑。最后,组织病理学评估表明,与纯GER相比,GER-UDCA-SLN的鼻腔给药不会损害鼻黏膜的结构完整性。结论,











































 京公网安备 11010802027423号
京公网安备 11010802027423号